What is organic synthesis:
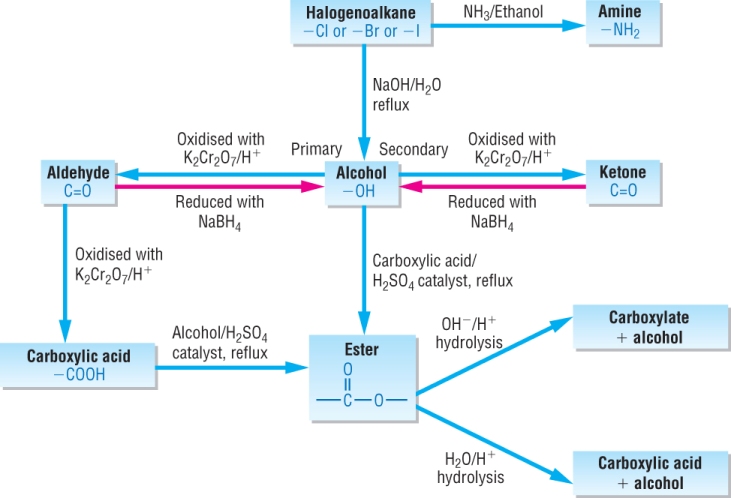
Synthetic routes in aliphatic / aromatic chemistry:
Aliphatic routes:

Aliphatic routes:
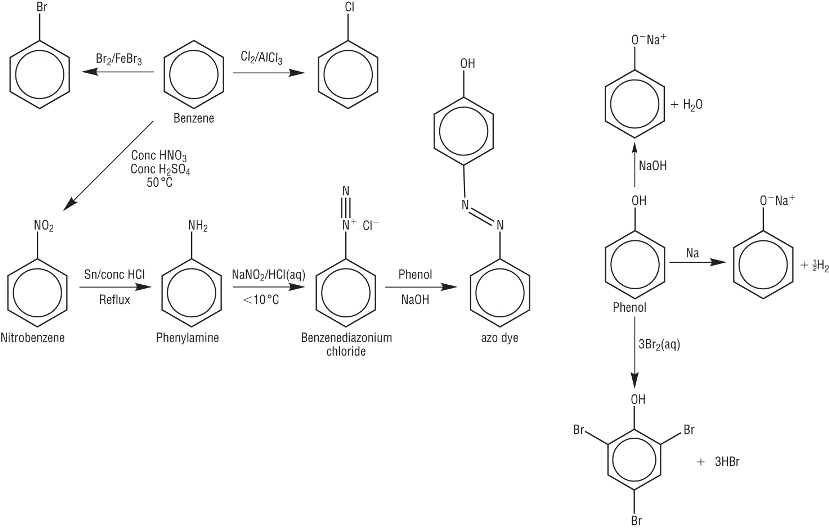
9.jpg) |
| Step 1:
|
|
| Step 2:
|
| Step 1:
|
|
| Step 2:
|
Task: Collate all your organic reactions diagrams from AS and A2 (include reagents and conditions)
Once you have completed the task aabove:
Have a go at synthesising:
Qu 1 P63 1,2 P65
|
|
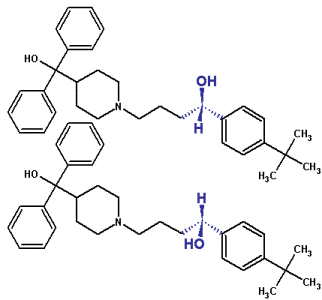
Mirror images cannot be superimposed upon each other.
These are called optical isomers:
|
|
Your hands are optical isomers of each other. Both are the same but are not super imposable.
A mirror image of one hand makes it possible to super impose it upon the other.
When a carbon atom has 4 different groups attached to it, you get 2 shapes that are mirror images of each other, known as optical isomers. The carbon atom is called the ‘Chiral Centre’.
The chiral carbons often have an asterisk on to show the chiral centre.
Compounds that do not have 4 different groups around a carbon atom are said to be achiral. (most compounds are these).
The 2 isomers are called enantiomers.
You must draw them in 3D and a mirror line is often drawn to help with this:
|
|
 |
|
| Synthesis | Nature | |
|
|
|
|
| Isomers | Both | one |
| Made | In the laboratory | In the body |
| Dose | Twice needed | Half needed |
| Side effects | Probably | None |
| Cost | Cheaper | More expensive |
| Separation in lab | Usual chemical difficult - isomers have same physical and chemical properties | Using enzymes - costly and time consuming |