A two step
synthesis of methyl 3-nitrobenzene
PROCEDURE
Safety
- Methanol is toxic and extremely flammable
Conc. Sulphuric acid is corrosive
Hydrocarbon solvent is flammable
Methyl benzoate is harmful
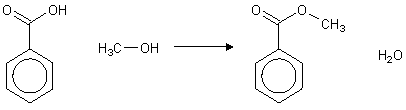
STEP 1: Formation of methyl benzoate
from benzoic acid:
|
|
- Add 8g of benzoic acid, 15cm3 of methanol
and 2cm3 of concentrated sulphuric acid to a 50cm3
pear shaped flask.
- Add 3-4 anti bumping granules and reflux for 45
minutes.
- Cool the mixture to room temperature and pour into a
separating funnel that contains 30cm3 of cold water.
- Rinse the pear shaped flask with 15cm3
hydrocarbon solvent and pour the washings into the separating funnel.
- Shake the flask releasing any gases regularly.
- Allow the 2 layers to separate and run off the lower
aqueous layer into a conical flask. (you don't need this solution).
- Add 15cm3 of water to your hydrocarbon
layer in the separating funnel and shake.
- Now add 15cm3 of 0.5M sodium carbonate
solution and shake again.
- Run off the aqueous layer again into a comical flask.
(you don't need this solution).
- Add a small amount of anhydrous sodium sulphate,
shake and filter out the sodium sulphate collecting the filtrate in a small
round bottom flask.
- Remove the hydrocarbon solvent by distilling it off.
Keep distilling and collect the methyl benzoate distillate above 190oC.
- Weigh your product.
