What is chromatography?
Chromatography is a separation technique first used to separate pigments in plant leaves.
There are 2 types of chromatography that you will study:
1) Thin Layer Chromatography - TLC
2) Gas Chromatography - GC

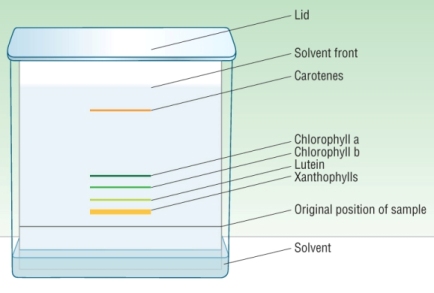
The plate is placed in a tank with the solvent below the sample.
The tank is covered and allowed to rise near the top of the plate.
The plate is removed, the solvent front marked and the solvent allowed to evaporate.
The data is analysed.
Chromatography is used in analysis but is more widely used as a method of separation.
How does chromatography work?
Chromatography works on the basis that components (molecules) have a different affinities for a stationary phase and for a mobile phase
Phases:
A) Stationary phase - is in a fixed place (paper in paper chromatography)
The molecules interact with the stationary phase slowing down their movement - ADSORPTION
B) Mobile phase - moved in a definite direction (water rises up in paper chromatography)
The molecules interact with the mobile phase speeding up their movement - SOLUBILITY
OVERALL - DIFFERENT MOLECULES MOVE AT DIFFERENT SPEEDS DUE TO THEIR DIFFERING INTERACTIONS WITH THE 2 PHASES
1) Thin Layer Chromatography - TLC
Solid stationary phase and liquid mobile phase
2) Gas Chromatography - GC
Liquid on solid support stationary phase and a gas mobile phase
Separation:
Is by adsorption or solubility depending on whether the stationary phase is a liquid or a solid:

Qu 1,2 P77
Thin - Layer Chromatography - TLC
Is used to check purity / monitor the extent of a reaction.
Phases:
A) Stationary phase - Silica gel, SiO2 or Aluminium oxide, Al2O3
B) Mobile phase - Solvent
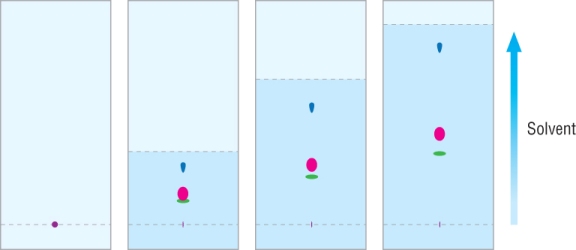
Producing the chromatogram:
1) Dissolve sample.
2) Draw a pencil line and spot sample using a capillary tube, allow to dry.
3) Place plate in a tank of solvent - solvent must be below line, seal the tank.
4) Separation is by adsorption - allow solvent to almost reach the top, draw a line here - solvent front.
5) Each separated component is a spot, if colourless use a UV lamp:
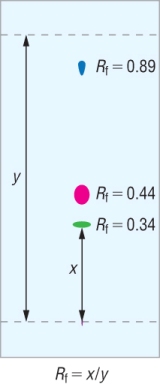
Rf values:
An Rf value shows how far a component has travelled compared with the solvent front:
 |
Rf | = | Distance moved by component | |||
| Distance moved by solvent front | ||||||
| Rf Green | = | 1.65 | 0.34 | |||
| 4.85 | ||||||
| Rf Pink | = | 2.15 | 0.44 | |||
| 4.85 | ||||||
| Rf Blue | = | 4.30 | 0.89 | |||
| 4.85 | ||||||
Rf is a ratio of the distance of the component moved : solvent front.
This means that the Rf values in this solvent will always be the same:
| For substances that are very soluble in the liquid, Rf will be close to | 1 |
| For substances that are not very soluble in the liquid, Rf will be close to | 0 |
Comparisons can also be made against pure components.
Limitations:
Similar compounds often have too similar Rf values.
Unknown compounds have no Rf value for comparison.
It is hard to find a solvent that will have the carrect amount of solubility - Goldilocks!!
Qu 1,2 P79
Is used to separate volatile compounds (gases) in a mixture.
The compounds, being volatile will have low boiling points (to evaporate easily
This process takes place in a gas chromatograph:
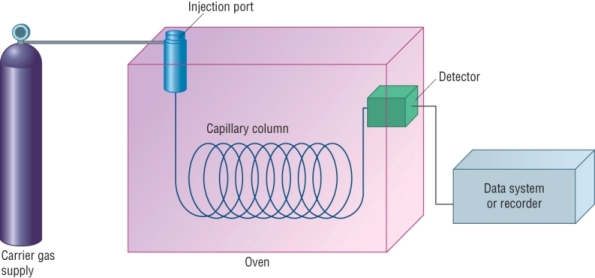
Producing the chromatograph:
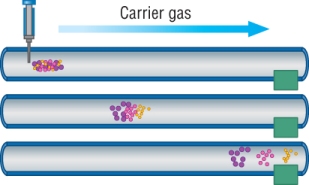
The mixture is injected into the chromatograph, it is vaporised and the mixture is carried through the column by the mobile carrier gas.
As the mixture flows through the capillary column, the components are slowed down by the stationary phase lining the column:
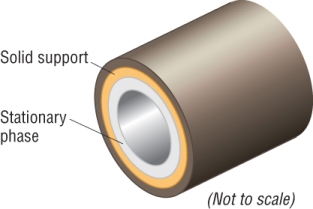 |
The stationary phase:
The mobile phase:
|
Different components are slowed down by different amounts - separation:
 |
|
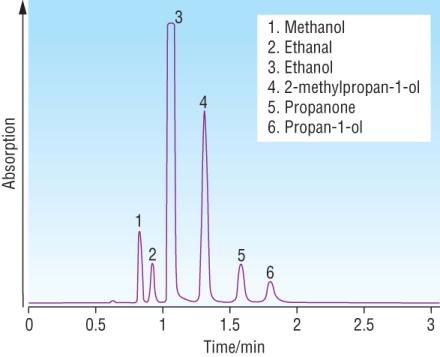
Retention time:
Is the time taken for a component to pass from inlet to detector.
Just like Rf values the retention time can be used to identify a component.
Known compounds will have known retention times at the same temperature, carrier gas and stationary phase.
The area under each peak (component) is equivalent to the amount of that component in the sample:
 |
Limitations of gas chromatography:
|
Qu 1,2 P81
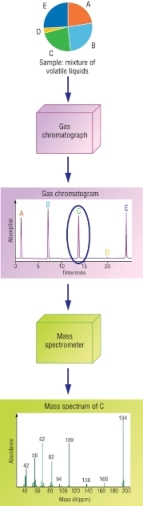
Gas Chromatography - Mass Spectroscopy - GC-MS
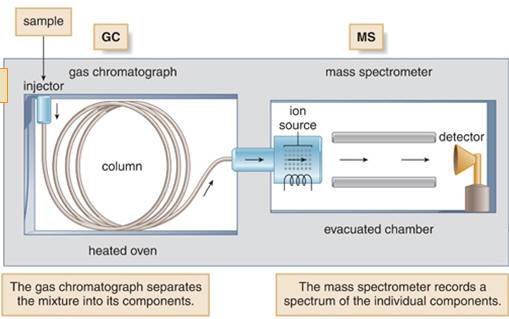
Combining gas chromatography with mass spectroscopy:
This is 2 techniques combined to provide a powerful analysis tool.
| Gas Chromatography, GC | Separates components |
| Mass Spectroscopy, MS | Gives detailed structural information |
 |
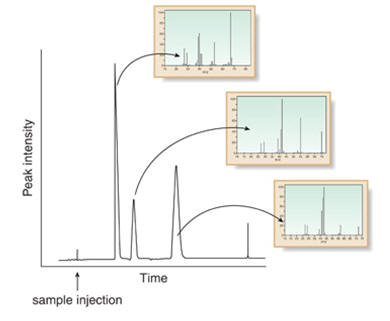 |
Each component is separated using GC then analysed using MS.
Summary:
 |
Uses for GC-MS 1) Forensics - scenes of crime 2) Environmental analysis - air pollutants, waste water, pesticides in food. 3) Airport security - explosives in luggage / airport security 4) Space probes - planetary atmospheres |
Qu 1-3 P83 Qu 1,2 P105 Qu 1 P107