Determining pKa from a pH titration curve for a weak
acid:
- It is possible to find out the pKa of a
weak acid from half neutralisation
- Lets use ethanoic acid and sodium hydroxide as an
example.
- The dissociation of the acid is:
| CH3COOH(l) |
D |
H+(aq) |
+ |
CH3COO-(aq) |
1 |
- Remember this acid only dissociates by a small amount
giving a small concentration of H+ and CH3COO-
ions.
- Look what happens during neutralisation:
| CH3COOH(l) |
+ |
OH-(aq) |
g |
CH3COO-(aq) |
+ |
H2O(l) |
2 |
- This increases the amount of CH3COO-
ions.
- If reaction 2 is increasing the amount of CH3COO-
ions it has an effect on reaction 1.
- Reaction 1 is now a buffer solution.
- This means that this equation still stands from
earlier:
| [H+] |
= |
Ka x |
[CH3COOH] |
| [CH3COO-] |
- Half way to neutral the 2 concentrations will be the
same: [CH3COO-]
= [CH3COOH]
- If they are the same then they will cancel out
meaning the hydrogen ion concentration is equal to the ka value:
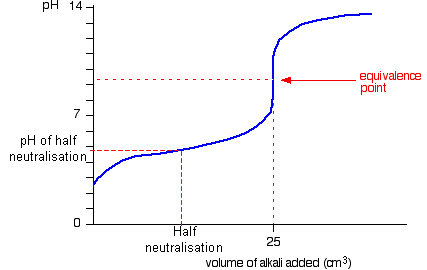
- This means that if you take the pH at half
neutralisation you can can calculate Ka
- Alternatively you can just read off a neutralisation
curve graph to
determine the pKa (later)
Fine tuning:
| [H+] |
= |
Ka x |
[CH3COOH] |
| [CH3COO-] |
- By fine tuning the [CH3COOH]
: [CH3COO-] ratio from
10 : 1 and from 1 : 10
you can control the buffer action by pH +/- 1
For the mathematicians -
Ignore this if you don't like it
- Look at this from the formula proved ealier:
| pH |
= |
pKa |
- log |
[CH3COOH] |
| [CH3COO-] |
- If the concentrations of the 2 species are equal
then -log 1 = 0