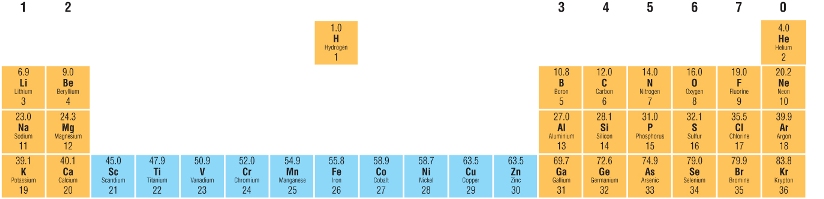

This means that Scandium and Zinc are not Transition elements.
It also explains why compounds of these are white and not coloured.
Sc 1s22s22p63s23p63d14s2 Sc3+ 1s22s22p63s23p6
Zn 1s22s22p63s23p63d104s2 Zn2+ 1s22s22p63s23p63d10
Writing electron configurations
Remember this is the arrangement of electrons in their orbitals.
Remember the 4s - sub shell fills before the 3d - sub shell:
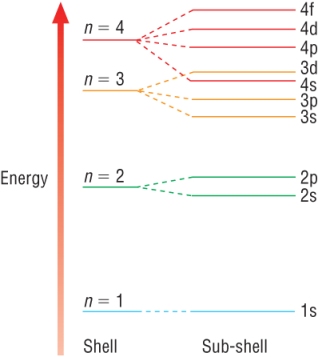
The electrons fill in order remember but their are 2 anomalous elements.
Chromium and Copper fills differently:
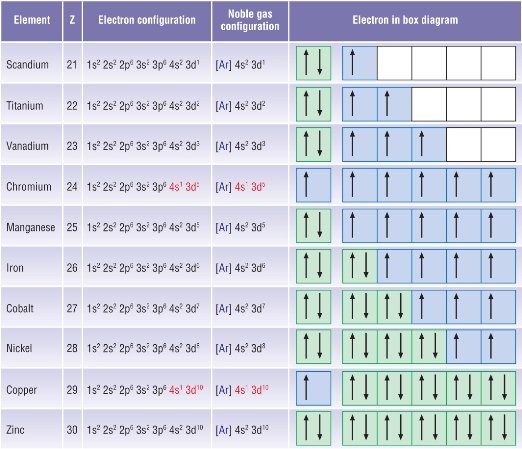 |
Cr: 1s22s22p63s23p63d44s2 (expected) Cr: 1s22s22p63s23p63d54s1 (actual) |
|
|
Cu: 1s22s22p63s23p63d94s2 (expected) Cu: 1s22s22p63s23p63d104s1 (actual)
|
This shows that these atoms show anomalous stability
A half - filled or full d - sub shell offers more stability than a full s - sub shell.
The electron configurations of ions:
As metals, the Transition elements lose electrons forming ions when they react.
When forming ions:
4s - sub shell empties before 3d - sub shell
This seems a bit odd as the 4s fills first.
When the orbitals have been filled the 4s and 3d sub shell levels swap over in the expected order.
This happens because the energy levels are very close in the first place.
The addition of electrons changes the energy levels slightly so they swap over (line emission spectra).
It is easier to do if you write the electron configuration in electron shell order:
Example 1
Fe: 1s22s22p63s23p63d64s2
Fe3+: 1s22s22p63s23p63d5
Example 2
Cu: 1s22s22p63s23p63d104s1
Cu2+: 1s22s22p63s23p63d9
Qu 1-2 P203
Properties of transition metal compounds
Physical properties - general metals
Shiny
High densities
High melting points and boiling points
Giant metallic structure
Delocalised electrons - good conductors of electricity
Physical properties - transition metals
Variable oxidation states
Coloured solutions
Catalysts - due to d shell electrons
Variable oxidation states
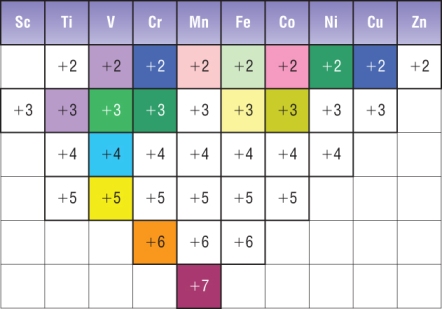 |
|
MAKE SURE YOU CAN WORK OUT OXIDATION STATES
Recap: Rules for assigning Oxidation Numbers
1) Ox. No. of an element = 0
2) Ox. No. of each atom in a compound counts separately. Sum = 0
3) Ox. No. of an ionic element = charge on ion.
4) In a polyatomic ion (SO42-), The sum of the Ox. No.’s of the atoms = charge on ion.
5) H = +1 except with metals (metal hydrides = -1).
6) Gp 7 (Halogens) = -1 (except with oxygen)
7) O = -2 except in peroxides (H2O2, O = -1)
Examples:
| 1) | KMnO4: | K | Mn | O x 4 | = | 0 |
| +1 | ? | 8- | = | 0 | ||
| +7 | ||||||
| 2) | K2Cr2O7: | K x 2 | Cr x 2 | O x 7 | = | 0 |
| +2 | 2 x ? | 14- | = | 0 | ||
| +6 |
Coloured compounds:
|
|
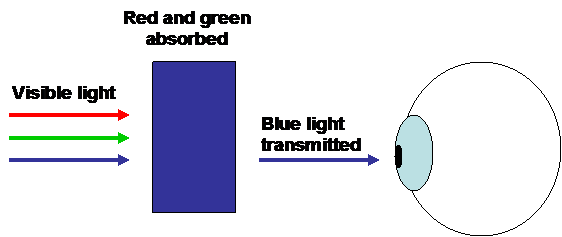 |
Sc3+: 1s22s22p63s23p6
No partially filled d shell = no colour. Sc3+ is white
Qu 1 - 3 P205
Transition metals as catalysts
1) Transferring electrons easily:
2) Providing a site to react:
|
|
Haber process:
| N2(g) | + | 3H2(g) | D | 2NH3(g) |
Contact process:
| 2SO2(g) | + | O2(g) | D | 2SO3(g) |
Hydrogenation of alkenes:

Decomposition of hydrogen peroxide:
| 2H2O2(aq) | à | 2H2O(l) | + | O2(g) |
Precipitating reactions
| Reaction | Notes | |||||
| Solution colour | Precipitate colour | |||||
|
Cu2+(aq) Pale blue |
+ |
2OH-(aq) |
à |
Cu(OH)2(s) Blue ppt |
|
|
|
Co2+(aq) Pink |
+ |
2OH-(aq) |
à |
Co(OH)2(s) Blue ppt |
u |
Turns beige in the presence of air |
|
Fe2+(aq) Green |
+ |
2OH-(aq) |
à |
Fe(OH)2(s) Green ppt |
u |
Turns brown at surface in the presence of air [O] |
|
Fe3+(aq) Yellow |
+ |
3OH-(aq) |
à |
Fe(OH)3(s) Rusty - brown ppt |
|
|
Qu 1 - 4 P207
Transition metals and complex ions
Complex ions
A property of transition metals is their ability to form complex ions:
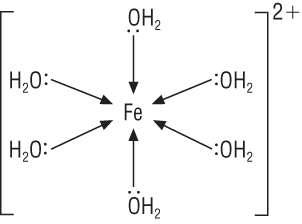 |
Complex ion: | Central metal ion is surrounded by ligands | |
| Ligand: | Molecule / ion which donates a pair of electrons forming a coordinate bond (dative bond) | ||
| Coordinate bond: | One of the bonded atoms has provided both electrons for the covalent bond |
In the example above:
Fe 2+ is the metal ion.
Ligands are the water molecules.
Coordination number is the number of coordinate bonds to the central metal ion = 6.
Square brackets groups the species and the overall charge is written outside the brackets.
Overall charge is the sum of the charges of the metal ion and the ligands (if the ligands have a charge)
Common ligands:
| Ligand | Formula | Charge |
| Water | :OH2 | 0 |
| Ammonia | :NH3 | 0 |
| Thiocyanate | :SCN- | -1 |
| Cyanide | :CN- | -1 |
| Chloride | :Cl- | -1 |
| Hydroxide | :OH- | -1 |
Ligands have a pair of electrons that donors which is used to make a dative covalent bond to the central metal ion.
All the ligands in the table have 1 lone pair and can form 1 dative covalent bond with the central metal ion.
These types of ligands are called monodentate.
The number of dative bonds a ligand is able to form is reflected in the prefix – mono, bi, hexa (poly) etc.
Examples:
1) [Ti(H2O)6]3+
| No Ligands x charge | + | No Ligands x charge | = | Total charge of ligands | + | Total charge on ion | = | Total charge on complex ion |
| 5 x 0 | + | - | = | 0 | + | ? | = | 3+ |
The central metal ion must be: Ti3+
2) [Co(H2O)5Cl]1+
| No Ligands x charge | + | No Ligands x charge | = | Total charge of ligands | + | Total charge on ion | = | Total charge on complex ion |
| 5 x 0 | + | 1 x 1- | = | 1- | + | ? | = | 2+ |
The central metal ion must be: Co2+
Shapes of complex ions:
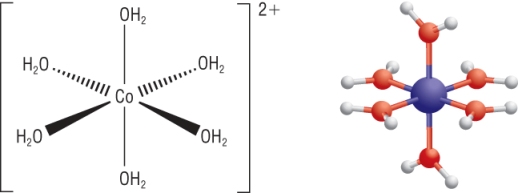
Qu 1 - 3
Stereoisomerism in complex ions
What is stereoisomerism?
They have the same structural formula but with a different spatial arrangement
This has been covered before in AS with:
Cis / Trans isomerism
Optical isomerism
Cis / Trans isomerism
These occur in:
1) Octahedral with 4 of one ligand and 2 of another ligand, [Co(NH3)4Cl2]+:
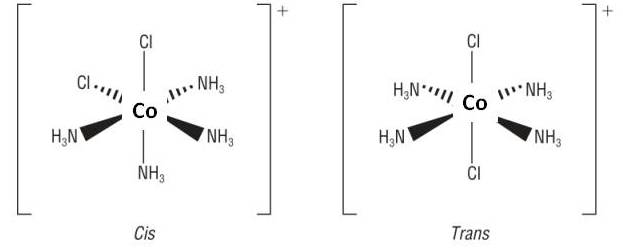 |
||||
| CIS = 2 ligands are at 90o to each other | TRANS = 2 ligands are at 180o to each other | |||
2) Square planar with 2 of one ligand and 2 of another ligand:
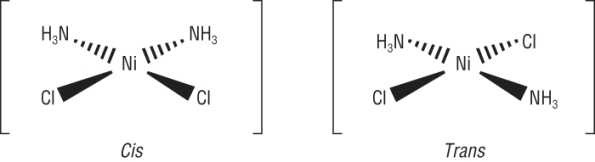 |
||||
| CIS = 2 ligands are at 90o to each other | TRANS = 2 ligands are at 180o to each other | |||
Transition metal complexes in medicine:
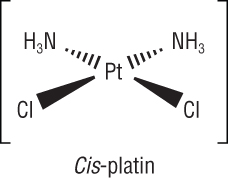 |
|
 |
|
Qu 1 - 2 P211
Bidentate and multidentate ligands
Bidentate ligands
Some ligands contain 2 lone pairs of electrons forming 2 coordinate bonds each.
Ethane - 1,2 - diamine, H2NCH2CH2NH2 abbreviated to 'en':
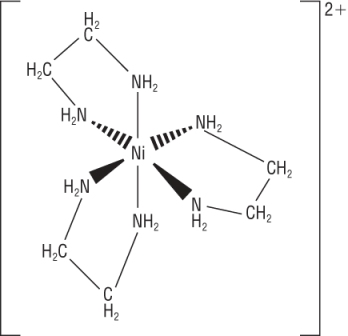 |
[Ni(en)3]2+
|
Cis - trans isomerism from bidentate ligands:
Bidentate ligands exhibit cis trans isomerism with an octahedral shape.
Ethanedioate ligand:
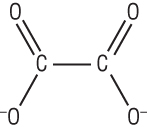
[Cr(C2O4)2(H2O)2]-
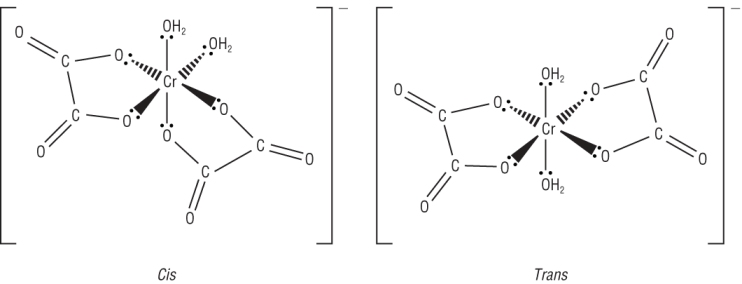 |
| CIS = 2 monodentate ligands are at 90o to each other | TRANS = 2 monodentate ligands are at 180o to each other |
Hexadentate ligand:
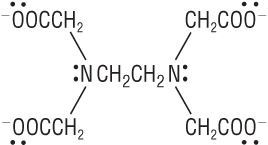 |
|
|
EDTA4- |
Optical isomers:
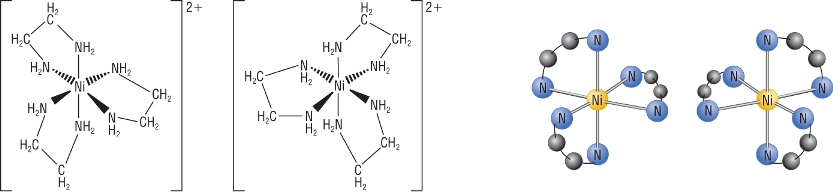
1) 3 bidentate ligands
2) 2 bidentate ligands / 2 monodentate ligands in the cis isomer only
3) Hexadentate ligand
Qu 1 P213
Ligand substitution in complex ions
Ligand substitution reactions
These are reactions when one ligand in a complex ion is substituted with another
1) The reaction of aq copper (II) ions and ammonia
| [Cu(H2O)6]2+(aq) | + | 4NH3(aq) |
D |
[Cu(NH3)4(H2O)2]2+(aq) | + | 4H2O(l) |
| Pale Blue sol | Deep Blue sol |
|
|
In practise:
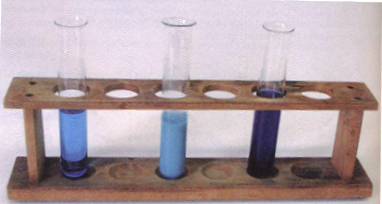 |
|
Ammonia contains:
| H2O(l) | + | NH3(aq) |
D |
NH4+(aq) | + | OH-(aq) |
a) Copper ions react with hydroxide ions:
|
Cu2+(aq) |
+ |
2OH-(aq) |
à |
Cu(OH)2(s) |
|
| Pale Blue | Pale Blue ppt |
|
b) Copper ions react with ammonia:
| [Cu(H2O)6]2+(aq) | + | 4NH3(aq) |
D |
[Cu(NH3)4(H2O)2]2+(aq) | + | 4H2O(l) |
| Pale Blue sol | Deep Blue sol |
Summary:
 |
|||
| [Cu(H2O)6]2+ | Cu(OH)2 | [Cu(NH3)4(H2O)2]2+ | |
2) The reaction of aq copper (II) ions and hydrochloric acid
| [Cu(H2O)6]2+(aq) | + | 4Cl-(aq) |
D |
[CuCl4]2-(aq) | + | 6H2O(l) |
| Pale Blue sol | Yellow sol |
|
|
|
3) The reaction of cobalt (II) ions and conc hydrochloric acid
| [Co(H2O)6]2+(aq) | + | 4Cl-(aq) |
D |
[CoCl4]2-(aq) | + | 6H2O(l) |
| Pink sol | Dark blue sol |
|
|
|
Qu 1-3 P215
Ligand substitution and stability constants
Haemoglobin and ligand substitution
Haemoglobin - is made from 2 parts
a) Haem: b) Globin:
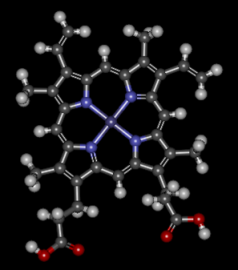 |
|
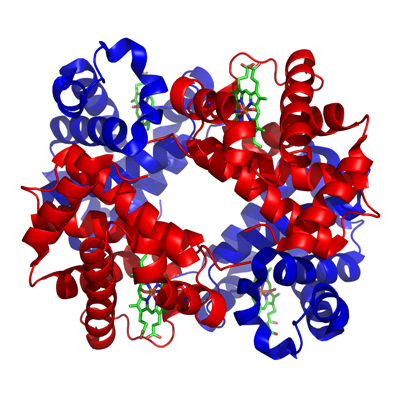 |
|
A simpler picture:
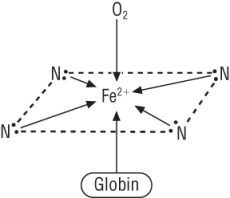 |
|
Carbon monoxide - the silent killer
Stability constants
|
|
Kc |
= |
[PRODUCTS]p | |
| [REACTANTS]r |
Example:
| [Co(H2O)6]2+(aq) | + | 4Cl-(aq) |
D |
[CoCl4]2-(aq) | + | 6H2O(l) |
| Kc |
= |
[ [CoCl4]2- ] [ [H2O] ]6 |
| [ [Co(H2O)6]2+ ] [ [Cl-] ]4 |
| Kc |
= |
[ [CoCl4]2- ] |
| [ [Co(H2O)6]2+ ] [ [Cl-] ]4 |
| Kstab |
= |
[ [CoCl4]2- ] |
| [ [Co(H2O)6]2+ ] [ [Cl-] ]4 |
The value of Kstab:
Example:
| [Cu(H2O)6]2+(aq) | + | 4Cl-(aq) |
D |
[CuCl4]2-(aq) | + | 6H2O(l) |
[ [Cu(H2O)6]2+ ] = 1.17 x 10-5 mol dm-3 and [ Cl-] = 0.08 mol dm-3 and Kstab = 4.17 x 10-5 mol-4 dm12
| Kstab |
= |
[ [CuCl4]2- ] |
| [ [Cu(H2O)6]2+ ] [ [Cl-] ]4 |
| 4.17 x 10-5 |
= |
[ [CuCl4]2- ] |
| 1.17 x 10-5 x 0.08 |
[ [CuCl4]2- ] = 2.00 mol dm-3
Qu 1 - 3 P217
Oxidation and reduction in transition metal chemistry
MnO4- and Fe2+
| Fe2+(aq) |
à |
Fe3+(aq) | + | e- | 1 |
| MnO4-(aq) | + | 8H+(aq) | + | 5e- |
à |
Mn2+(aq) | + | 4H2O(l) | 2 |
| MnO4-(aq) | + | 8H+(aq) | + | 5Fe2+(aq) |
à |
Mn2+(aq) | + | 4H2O(l) | + | 5Fe3+(aq) |
| Purple | Colourless |
Carrying out redox titrations
| MnO4-(aq) | + | 8H+(aq) | + | 5Fe2+(aq) |
à |
Mn2+(aq) | + | 4H2O(l) | + | 5Fe3+(aq) |
| Purple | Colourless |
Worked example:
25.0cm3 of a solution of iron (II) salt required 23.00cm3 of 0.0200 mol dm-3 potassium manganate (VII) for complete oxidation in acidic solution.
| MnO4-(aq) | + | 8H+(aq) | + | 5Fe2+(aq) |
à |
Mn2+(aq) | + | 4H2O(l) | + | 5Fe3+(aq) |
| 23cm3 | 1 : 5 | 25cm3 | ||||||||
| 0.02 mol dm-3 | Conc = Mol x 1000/v | |||||||||
|
|
= 2.3 x 10-3 x 1000/25 |
|||||||||
| = 0.092 mol dm-3 | ||||||||||
|
|
||||||||||
| Moles = C x V/1000 | ||||||||||
| = 0.02 x 23/1000 |
|
|||||||||
| = 4.6 x 10-4 | x 5 | = 2.3 x 10-3 | ||||||||
Qu 1 - 2 P 219
1) Calculate the Mr and formula of an iron (II) salt
2) Calculating the % mass of iron in iron tablets
3) Calculating the purity of iron samples
4) Applying your knowledge to unfamiliar redox reactions / titrations
1) Calculate the Mr and formula of an iron (II) salt
2.950g of hydrated iron (II) sulphate, FeSO4.xH2O, was dissolved in 50cm3 of sulphuric acid. This was made up to 250cm3 with distilled water.
25cm3 of this was titrated with 0.01 mol dm-3 KMnO4 and 21.20cm3of this was used
| MnO4-(aq) | + | 8H+(aq) | + | 5Fe2+(aq) |
à |
Mn2+(aq) | + | 4H2O(l) | + | 5Fe3+(aq) |
| 21.20cm3 | 25cm3 | |||||||||
| 0.01 mol dm-3 |
(misprint in book) |
|||||||||
|
|
||||||||||
| Moles = C x V/1000 | 1 : 5 | |||||||||
| = 0.01 x 21.20/1000 |
|
|||||||||
| = 2.12 x 10-4 | x 5 | = 1.06 x 10-3 | ||||||||
2) Calculating the % mass of iron in iron tablets
A multivitamin tablet has a mass of 0.325g and contains iron. The powdered tablet was dissolved in some water and sulphuric acid.
12.10cm3 of 0.002 mol dm-3 KMnO4 was titrated until the first permanent pink colour.
| MnO4-(aq) | + | 8H+(aq) | + | 5Fe2+(aq) |
à |
Mn2+(aq) | + | 4H2O(l) | + | 5Fe3+(aq) |
| 12.10cm3 | Mass = moles x Ar | |||||||||
| 0.002 mol dm-3 |
= 1.21 x 10-4 x 55.8 |
|||||||||
|
|
= 0.00675g | |||||||||
|
|
||||||||||
| Moles = C x V/1000 | 1 : 5 | |||||||||
| = 0.002 x 12.10/1000 |
|
|||||||||
| = 2.42 x 10-5 | x 5 | = 1.21 x 10-4 | ||||||||
4) Applying your knowledge to unfamiliar redox reactions / titrations
25cm3 portion of H2O2 was made up to 250cm3 with distilled water.
25cm3 portions of this was acidified and titrated with 0.0200 mol dm-3 KMnO4, 38.00cm3 of this was required to completely oxidise the hydrogen peroxide.
Calculate the original concentration of the hydrogen peroxide. The following equation represents the oxidation of hydrogen peroxide
| H2O2(aq) |
à |
O2(g) | + | 2H+ | + | 2e- | 1 |
| MnO4-(aq) | + | 8H+(aq) | + | 5e- |
à |
Mn2+(aq) | + | 4H2O(l) | 2 |
| 2MnO4-(aq) | + | 16H+(aq) | + | 5H2O2(aq) |
à |
2Mn2+(aq) | + | 8H2O(l) | + | 5O2(aq) | + | 10H+(aq) |
| 2MnO4-(aq) | + | 6H+(aq) | + | 5H2O2(aq) |
à |
2Mn2+(aq) | + | 8H2O(l) | + | 5O2(aq) |
| Purple | Colourless |
25cm3 portion of H2O2 was made up to 250cm3 with distilled water.
25cm3 portions of this was acidified and titrated with 0.0200 mol dm-3 KMnO4, 38.00cm3 of this was required to completely oxidise the hydrogen peroxide.
Calculate the original concentration of the hydrogen peroxide. The following equation represents the oxidation of hydrogen peroxide
| 2MnO4-(aq) | + | 6H+(aq) | + | 5H2O2(aq) |
à |
2Mn2+(aq) | + | 8H2O(l) | + | 5O2(aq) |
| 38.00cm3 | 25cm3 | ||||||||
| 0.02 mol dm-3 |
C = moles x 1000 / V |
||||||||
|
|
= 1.9 x 10-3 x 1000 / 25 | ||||||||
|
= 0.0760 mol dm-3 |
|||||||||
|
|
|||||||||
| Moles = C x V/1000 | 2 : 5 | ||||||||
| = 0.02 x 38.00/1000 |
|
||||||||
| = 7.6 x 10-4 | x 2.5 |
= 1.9 x 10-3 |
|||||||
Qu 1 - 2 P221
Redox titrations - iodine and thiosulphate
| 2S2O32-(aq) | + | I2(aq) |
à |
2I-(aq) | + | S4O62-(aq) |
| BROWN | COLOURLESS |
1) Iodine is liberated from it ions - using an oxidising agent (redox reaction):
2I- à I2 + 2e-
a) Copper (II): Cu2+
b) Dichromate: Cr2O72-
c) Chlorate: ClO-
2) The liberated iodine is titrated with a known concentration of thiosulphate,
| 2S2O32-(aq) | + | I2(aq) |
à |
2I-(aq) | + | S4O62-(aq) |
| BROWN | COLOURLESS |
Example:
30cm3 of bleach was added to an excess of iodide ions, I- in sulphuric acid.
Bleach contains chlorate (I) ions, ClO-.
In the presence of acid, chlorate (I) ions oxidise iodide ions, I- to iodine, I2.
The iodine formed was titrated against 0.200 Mol dm-3 of sodium thiosulphate, 29.45cm3 was required.
Calculate the concentration of chlorate ions, ClO- in the bleach:
| ClO-(aq) | + | 2H+(aq) | + | 2I-(aq) |
à |
Cl-(aq) | + | I2(laq) | + | H2O(aq) | [1] |
| 30.00cm3 | BROWN | ||||||||||
Then:
| 2S2O32-(aq) | + | I2(aq) |
à |
2I-(aq) | + | S4O62-(aq) |
| BROWN | COLOURLESS | |||||
| 0.200 Mol dm-3 | ||||||
| 29.45 cm3 |
|
|
|||||||||
|
|
|||||||||
| Moles = C x V/1000 | 2 : 1 | ||||||||
| = 0.200 x 29.45/1000 |
|
||||||||
| = 5.890 x 10-3 | x 0.5 |
= 2.945 x 10-3 |
|||||||
Estimating the copper content of solutions and alloys:
1) Iodine is liberated from it ions - using Cu2+ ions:
| 2Cu2+(aq) | + | 4I- 2(aq) |
à |
2CuI(s) | + | I2(aq) |
| COLOURLESS | WHITE SOLID | BROWN SOLn |
2) The liberated iodine is titrated with a known concentration of thiosulphate,
| 2S2O32-(aq) | + | I2(aq) |
à |
2I-(aq) | + | S4O62-(aq) |
| BROWN | COLOURLESS |
Copper metal:
1) Convert Cu to Cu2+:
| conc. HNO3 | ||||||||
| Cu(s) |
à |
Cu2+(aq) | + | 2e- | [1] | |||
2) Liberate iodine:
| 2Cu2+(aq) | + | 4I- 2(aq) |
à |
2CuI(s) | + | I2(aq) |
| COLOURLESS | WHITE SOLID | BROWN SOLn |
3) Titrate with a known concentration of thiosulphate,
| 2S2O32-(aq) | + | I2(aq) |
à |
2I-(aq) | + | S4O62-(aq) |
| BROWN | COLOURLESS |
Ratio: S2O32- : I2 : Cu2+ : Cu
2 : 1 : 2 : 2
Overall ratio is 1 : 1
Example:
0.500g of bronze was reacted with nitric acid giving a Cu2+ solution.
The solution was reacted with iodide ions, I- forming a solution of iodine, I2.
This iodine solution, I2 was then titrated with 0.200 mol dm-3 solution of sodium thiosulphate, S2O32-. 22.40cm3 of this was required.
Calculate the % copper in brass:
1) Convert Cu in brass to Cu2+:
| conc. HNO3 | ||||||
| Cu(s) |
à |
Cu2+(aq) | + | 2e- | [1] |
2) Liberate iodine:
| 2Cu2+(aq) | + | 4I- 2(aq) |
à |
2CuI(s) | + | I2(aq) |
| COLOURLESS | WHITE SOLID | BROWN SOLn |
3) Titrate with a known concentration of thiosulphate,
| 2S2O32-(aq) | + | I2(aq) |
à |
2I-(aq) | + | S4O62-(aq) |
| BROWN | COLOURLESS | |||||
| 0.200 Mol dm-3 | ||||||
| 22.4 cm3 |
|
|
|||||||||
|
|
|||||||||
| Moles = C x V/1000 | |||||||||
| = 0.200 x 22.40/1000 | |||||||||
| = 4.48 x 10-3 | |||||||||
Qu 1 - 2 P 223 / Qu 9 P 225 / Qu's 226 - 229