In AS we looked at ionic bonding and learned that:
Ionic bonds are strong
Due to strong electrostatic forces of attraction
This means that the melting and boiling points of ionic compounds are very high.
This is due to the lattice enthalpy, DH qLE:
Definition – Lattice enthalpy
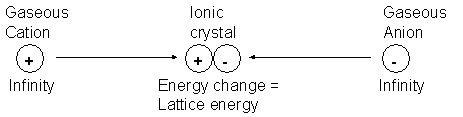
The lattice enthalpy is the enthalpy change when 1 mole of an ionic compound is formed from its gaseous ions under standard conditions (298K, 100kPa).
|
|
For sodium chloride:
| Na+(g) | + | Cl-(g) | g | NaCl(s) | DHqLE | = | -781 KjMol-1 |
Features of lattice enthalpy:
 |
|
As it is not possible to calculate directly, a special type of Hess's cycle is used.
This is called a Born - Haber cycle.
These cycles require many more types of enthalpy changes:
Key enthalpy changes:
1) Standard enthalpy change of formation, DH qf :
1 mole of compound is formed from its constituent elements in their standard state.
Example - usually exothermic
K(s) + 1/2Cl2(g) à KCl(s) DH qf = - 437 KJ Mol-1
2) The standard enthalpy change of atomisation,DH qat :
1 mole of gaseous atoms are formed from the element in its standard state.
Example - Always endothermic as bonds have to be broken:
Mg(s) à Mg(g) DH qat = +148 KJ Mol-1
3) First ionisation energy, DH qI1 :
1 mole of gaseous 1+ ions is formed from gaseous atoms
Example - Always endothermic as an electron has to overcome the attraction from the nucleus:
Li(g) à Li+(g) + e- DH qI1 = + 520 KJ Mol-1
4) Second ionisation energy, DH qI2 :
1 mole of gaseous 2+ ions is formed from 1 mole gaseous 1+ ions
Example - Always endothermic as an electron has to overcome the attraction from the nucleus:
Ca1+(g) à Ca2+(g) + e- DH qI2 = + 1145 KJ Mol-1
5) First Electron affinity, DH qEA1 :
1 mole of gaseous 1- ions formed from gaseous atoms
Example - Is exothermic as an electron is attracted to the outer shell by the nucleus:
Cl(g) + e- à Cl-(g) DH qEA1 = - 349 KJ Mol-1
6) Second Electron affinity, DH qEA2 :
1 mole of gaseous 2- ions formed from 1 mole gaseous 1- ions
Example - Is endothermic as the electron being put in is repelled by the 1- ion:
O-(g) + e- à O2-(g) DH qEA2 = - 798 KJ Mol-1
Qu 1 - 3 P 167
Constructing Born - Haber cycles
Born - Haber cycles are basically a form of Hess's cycle particularly for calculating lattice enthalpies.
Start with the elements at 'zero energy' - called the datum line.
Endothermic processes go up
Exothermic processes go down
A simple view of the Born - Haber cycle is shown below:
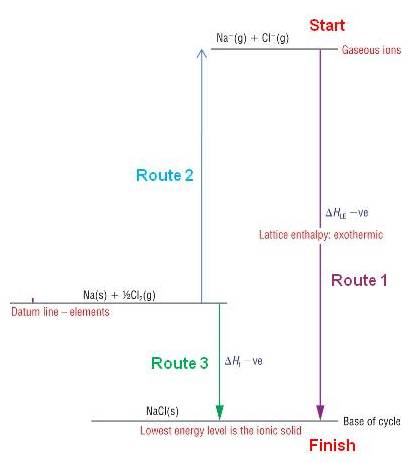 |
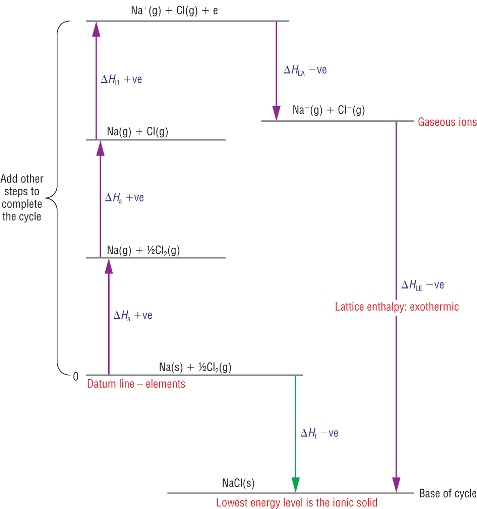
Route 2 is actually a multi - step process changing elements to gases then to ions
 |
 |
Route 2 has to be calculated in stages using a combination of enthalpy changes.
Taking one element at a time use enthalpy changes to convert each element into gaseous atoms.
From there, use successive ionisation energies or electron affinities to convert the gaseous atoms into gaseous ions.
Apply Hess’s cycle to calculate the Lattice energy.
Calculation:
Route 1 = - Route 2 + Route 3
Route 1 = Route 3 - Route 2
Lattice enthalpy, DHqLE = DHqf - Route 2 (the rest)
Or better - rearranged:
| DHqLE | = | DHqf | - | (SUM OF DHq THE REST) |
Other rules:
2 ions of the same formula, MgCl2 – You need to count the enthalpies from the atom in its standard state to the ion in its gaseous state TWICE.
More than 1 charge on the ion – you need to 2 ionisation energies or electron affinities:
eg Mg à Mg+ à Mg2+ or O à O- à O2-
Qu 1 - 3 P169
Born - Haber cycle calculation
Worked examples:
1) Caesium chloride:
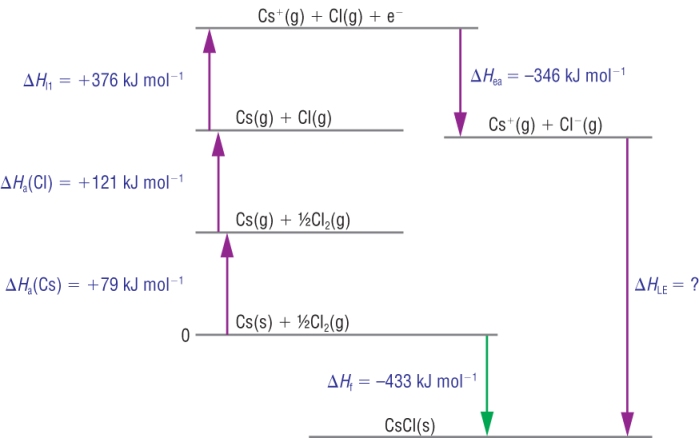
Calculation:
| DHqLE | = | DHqf | - | (SUM OF DHq THE REST) | |
| DHqLE | = | - 433 | - | ( +79 + 121 + 376 + - 346) | |
| DHqLE | = | - 433 | - | ( +79 + 121 + 376 - 346) | |
| DHqLE | = | - 663 |
Kj mol-1 |
||
2) Sodium oxide:
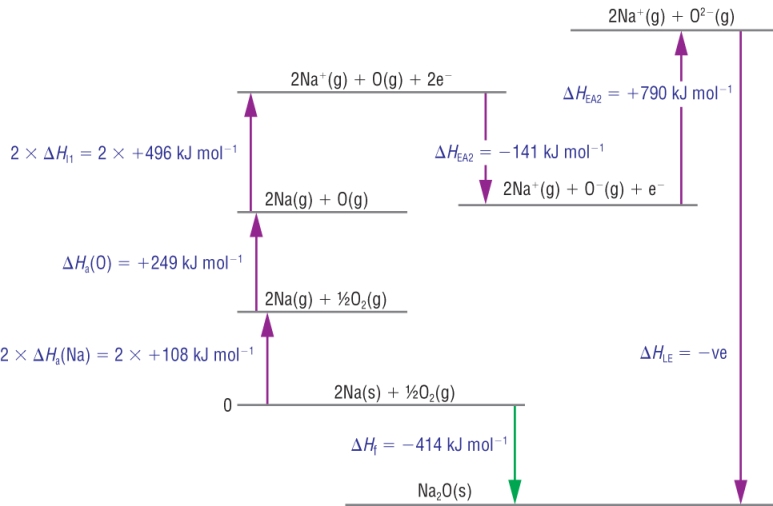
Calculation:
| DHqLE | = | DHqf | - | (SUM OF DHq THE REST) | |
| DHqLE | = | - 414 | - | ( +216 + 249 + 992 + - 141 + 790) | |
| DHqLE | = | - 414 | - | ( +216 + 249 + 992 - 141 + 790) | |
| DHqLE | = | - 2520 |
Kj mol-1 |
||
Qu 1 P 171
Further examples of Born - Haber cycles
3) Calcium chloride:
These cycles can be used to calculate other unknowns:
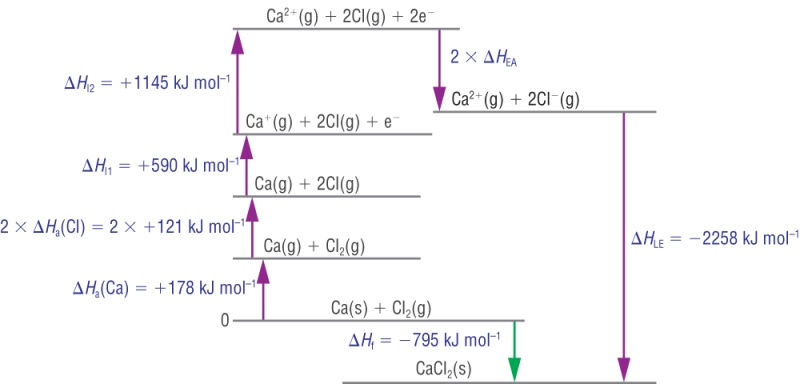
Calculation:
| DHqLE | = | DHqf | - | (SUM OF DHq THE REST) | |
| -2258 | = | - 795 | - | ( +178 + 242 + 590 + 1145 + (2 x DHqEA)) | |
| -2258 | = | - 795 | - | (+ 2155 + (2 x DHqEA)) | |
| -2258 | = | - 795 | - | 2155 - (2 x DHqEA) | |
| -2258 | = | - 2950 | - (2 x DHqEA) | ||
| 2 x DHqEA | = | - 2950 | + 2258 | ||
| 2 x DHqEA | = | - 692 | |||
| DHqEA | = | - 346 |
Kj mol-1 |
||
4) Copper (II) oxide:
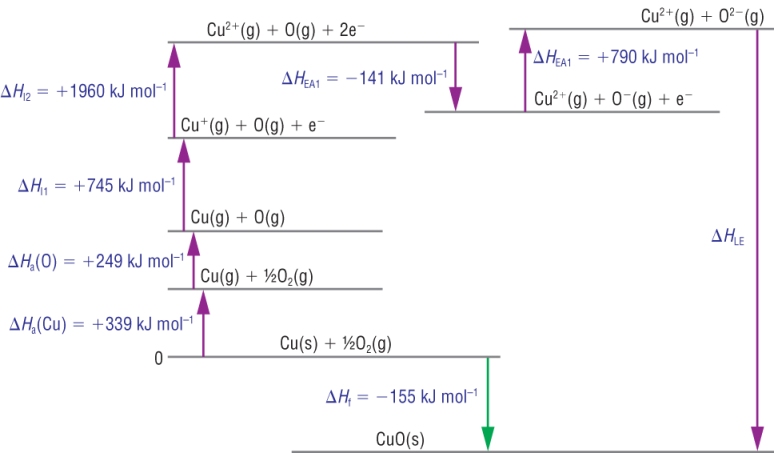
Calculation:
| DHqLE | = | DHqf | - | (SUM OF DHq THE REST) | |
| DHqLE | = | - 155 | - | ( +339 + 249 + 745 + 1960 + -141 + 790) | |
| DHqLE | = | - 155 | - | ( +339 + 249 + 745 + 1960 - 141 + 790) | |
| DHqLE | = | - 4097 |
Kj mol-1 |
||
Qu 1 P173
When ionic compounds dissolve in water the ions separate.
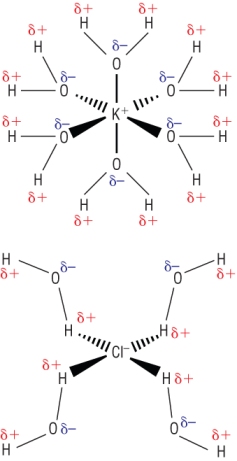 |
|
1) Enthalpy change of solution, DHqs
1 mole of a compound is dissolved in water under standard conditions
Example - This process can be endothermic or exothermic:
KCl(s) + aq à K+(aq) + Cl-(aq)
2) Enthalpy change of hydration, DHqhyd
1 mole of aqueous ions are formed from their gaseous ions under standard conditions
Example - This process is an exothermic process:
K+(g) + aq à K+(aq)
Cl-(g) + aq à Cl-(aq)
What happens when a solid dissolves:
In terms of a Hess's cycle:
1) Ionic lattice breaks down into gaseous ions, Ein:
This is the opposite of the lattice enthalpy:
| K+(g) | + | Cl-(g) | g | KCl(s) | DHqLE | = | - 771 KjMol-1 |
| KCl(s) | g | K+(g) | + | Cl-(g) | DHqLE | = | + 771 KjMol-1 |
2) Hydration of gaseous ions, Eout:
K+(g) + aq à K+(aq)
Cl-(g) + aq à Cl-(aq)
The resulting enthalpy change is known as the enthalpy change of solution
Calculating a lattice enthalpy from enthalpy changes of solution and hydration:
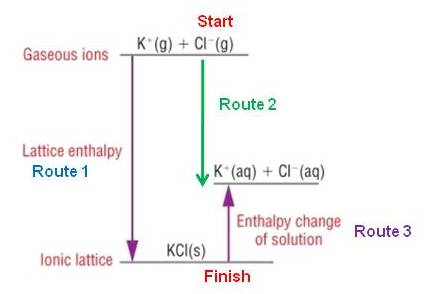 |
Route 1 =
Route 2 +
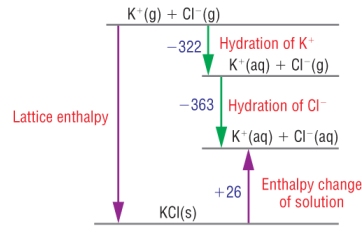
- Route 3 Route 1 = Route 2 - Route 3 DHqLE = Route 2 - DHqs
DHqLE = (SUM OF DHqhyd ions) - DHqs DHqLE = (- 322 + - 363) - +26 DHqLE = (- 322 - 363) - 26 DHqLE = - 685 - 26 DHqLE = - 711 Kj mol-1 |
 |
Qu 1 P175
Understanding hydration and lattice enthalpies
Lattice enthalpies
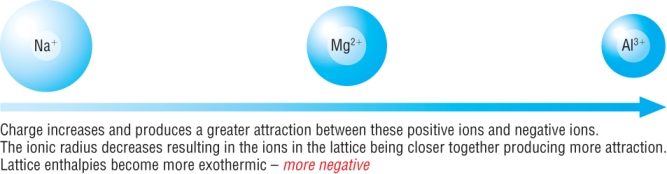
Lattice enthalpy is due to strong electrostatic forces of attraction between oppositely charged ions.
This means that 2 things can effect the value of the lattice enthalpy:
1. Ionic size
2. Ionic charge
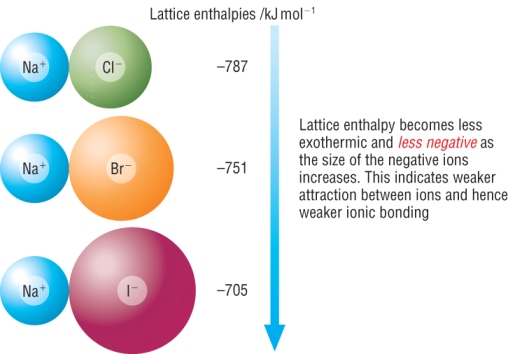
Ionic size
Small ions can pack together closer.
This gives a stronger electrostatic force of attraction.
As ions become larger down a group, the attraction decreases.
This means that the lattice enthalpy will be less exothermic:
Ionic charge
The larger the charge on the ion, the stronger electrostatic force of attraction between the ions.
As the ionic charge increases, the electrostatic forces of attraction also increases.
This means that the lattice enthalpy will be more exothermic:
Enthalpy change of hydration:
Enthalpy change of hydration is due to attractive forces between ions and water.
This means that 2 things can effect the value of the lattice enthalpy:
1. Ionic size
2. Ionic charge
Ionic size
Small ions attract water molecules closer.
This gives a stronger force of attraction.
As ions become larger down a Group, the attraction decreases.
This means that the hydration enthalpy will be less exothermic:
|
Down the group, size increases, DHqhyd gets less exothermic |
Ion | DHqhyd |
| Cl- | -363 | |
| Br- | -336 | |
| I- | -295 |
Ionic charge
A greater charge on ions attract water molecules stronger.
This gives a stronger force of attraction.
The ions also become smaller across a Period, the attraction increases further.
This means that the hydration enthalpy will be more exothermic:
|
Across a Period, charge increases and size decreases, DHqhyd gets more exothermic |
Ion | DHqhyd |
| Na+ | -406 | |
| Mg2+ | -1921 | |
| Al3+ | -4665 |
Qu 1 - 3 P177
What is entropy:
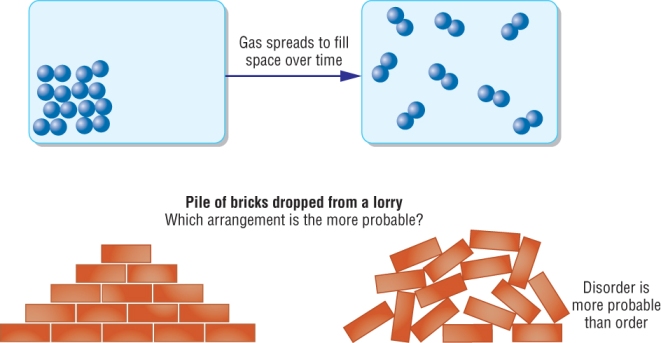
Entropy is a measure of disorder in a system.
There is always some degree of disorder as particles always have some sort of motion, so values are always positive.
As motion is related to enthalpy (energy) then the 2 are related.
As entropy is due to disorder and so enthalpy (energy) it can be described as the dispersal of energy.
Entropy:
Melting ice cream
Smell of cooking spreading
These are occasions when natural processes lead to disorder.
There is a tendency in nature for processes to lead to disorder:
Your bedroom
Expanding Universe
What can entropy tell us?
All these processes start off ordered and become disordered
The entropy starts of with a low value (ordered) and leads to a higher value (disordered)
This means that reactants and products will have an entropy content.
As Chemists we are interested in the change in entropy of a chemical system.
This gives us an idea as to whether a reaction will occur or not
|
Order |
State |
Explanation |
Entropy |
|
|
Ordered (O) |
Solids |
Vibrating in fixed position |
Low |
|
|
Disordered (DO) |
Liquids/ Aqueous |
Free moving but closely packed |
Medium |
|
|
Very Disordered (VDO) |
Gases |
As far apart as possible, moving lots |
High |
|
Using Entropy:
| Reaction | NH4NO3(s) | + | aq | à | NH4+(aq) | + | NO3-(aq) |
| Order | Ordered | Disordered | à | Disordered | Disordered | ||
| Overall | Ordered | à | Disordered | ||||
| LIKELY TO HAPPEN | |||||||
| Reaction | MgCO3(s) | à | MgO(s) | + | CO2(g) |
| Order | Ordered | à | Ordered | Very disordered | |
| Overall | Ordered | à | Disordered | ||
| LIKELY TO HAPPEN | |||||
Calculating entropy:
Standard entropy content of a substance can be looked up in a data book.
They have the units J Mol K-1
The difference in entropy content between the products and reactants will give a value.
If the value is positive it means that the system is moving to more disorder - LIKELY TO HAPPEN
If the value is negative it means that the system is moving towards more order - UNLIKELY TO HAPPEN
Worked example:
For the reaction:
| Reaction | N2(g) | + | 3H2(g) | à | 2NH3(g) |
| Entropy content | + 192 | + 131 | + 193 |
Qu 1 - 2 P179
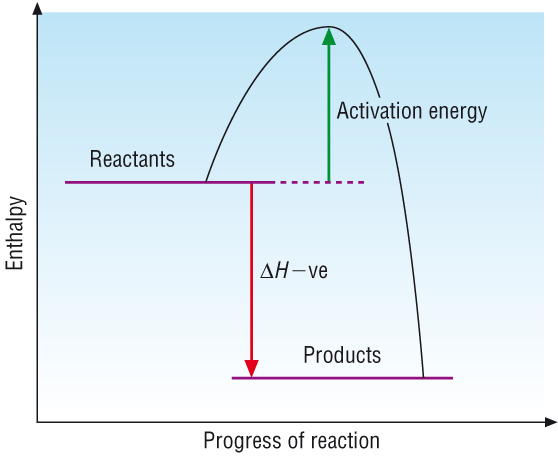
Spontaneous process
Spontaneous reactions occur on their own and move to products with a lower energy and more stability.
Exothermic reactions:
 |
|
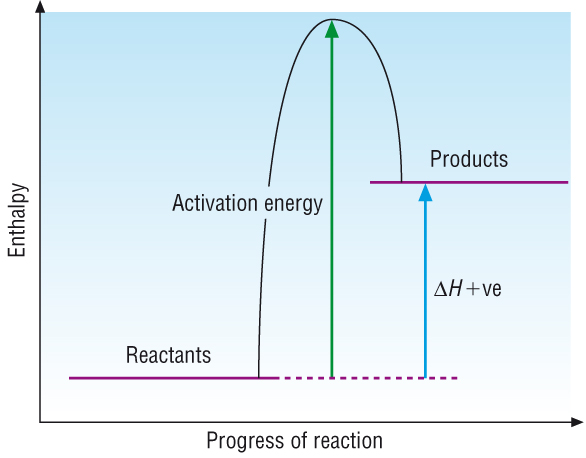
Endothermic reactions:
 |
|
| Enthalpy | Deals with the contribution of energy of the surroundings | |
| Entropy | Deals with the contribution of energy of the system |
Energy from entropy
Energy derived from entropy = TDS
Free energy and feasibility:
| 1 | Temperature in k | T |
| 2 | Entropy change of the system | DS |
| 3 | Enthalpy change with the surroundings | DH |
| DG | = | DH | - | TDS |
| Surroundings | System |
DG < 0 Negative
| DH | DS | DG | Feasibility / spontaneous |
| (-)ve | (+)ve | (-)ve | Feasible |
| (+)ve | (-)ve | (+)ve | Never feasible |
| (-)ve | (-)ve | (-)ve at low temps | Feasible at low temps |
| (+)ve | (+)ve | (-)ve at high temps | Feasible at high temps |
How do endothermic reactions take place:
| DG | = | DH | - | TDS |
| DG | = | DH | - | TDS |
| DH | < | TDS |
| (+)ve | < | TDS |
Example:
At what temperature will ZnCO3 decompose?
| Reaction | ZnCO3(s) | à | ZnO(s) | + | CO2(g) | DH = +71 KJ mol-1 |
| Entropy, DS | +82 | +44 | +214 |
1) Calculate DSqsystem:
2) Assume DG = 0 to calculate T:
| DG | = | DH | - | TDS |
| 0 | = | + 71 | - | T x 0.176 |
| T x 0.176 | = | + 71 |
| T | = | + 71 |
| 0.176 |
| T | = | 403 k | (-273 for oC) |
| T | = | 130 oC |
Qu 1 - 2 P181 / 1 - 4 P195 / 1 - 7 P196 - 198