| Type of Bonding | Which types of atoms combine | Electrons |
| Ionic | Metal + Non-metal | Transferred: Metal à Non-metal |
| Covalent | Non-metal + Non-metal | Shared: Between the atoms |
| Metallic | Metal + Metal | Shared: Between all atoms |
| 2Na | à | 2Na+ | + | 2e- |
| 1s22s22p63s1 | à | 1s22s22p6 |
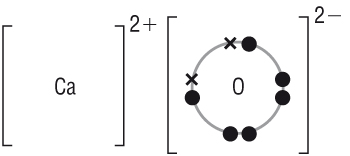
| O | + | 2e- | à | O2- |
| 1s22s22p4 | 1s22s22p6 |
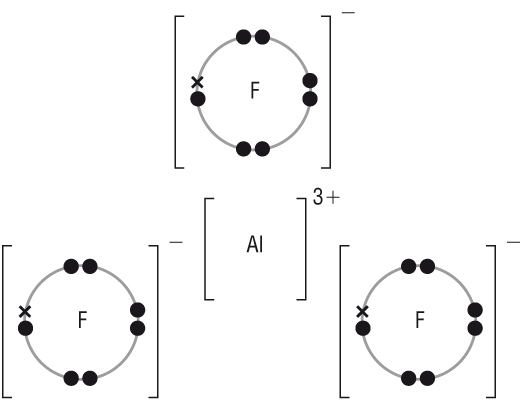
| CaO | AlF3 | |||||||||||
 |
 |
|||||||||||
|
|
|||||||||||
|
|
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 0 |
| No e's in outer shell | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Full |
| No electrons lost gained | Lose 1 | Lose 2 | Lose 3 | x | Gain 3 | Gain 2 | Gain 1 | x |
| Charge on ion | 1+ | 2+ | 3+ | x | 3- | 2- | 1- | x |
Transitions metals:
| Element | Charge on ion / oxidation number | ||||||
| Mn | 2+ | 3+ | 4+ | 5+ | 6+ | 7+ | |
| Roman numeral | II | III | IV | V | VI | VII | |
Iron (II) Fe2+
Copper (II) Cu2+
Molecular ions (common ions)
| 1+ | 1- | 2- | 3- | ||||
| Ammonium | NH4+ | Hydroxide | OH- | Carbonate | CO32- | phosphate | PO43- |
| Nitrate | NO3- | Sulphate | SO42- | ||||
| Nitrite | NO2- | Sulphite | SO32- | ||||
| Hydrogen carbonate | HCO3- | Dichromate | Cr2O72- | ||||
The ions in a chemical formula must add up to zero.
Use subscripts after an ion in a formula to double/triple that ion so the sum=0. eg. CuCl2
If you are double/tripling ions that consist of more than one element (molecular ions) brackets must be used. eg. Ca(OH)2
If Roman numeral numbers follow a transition metal ion in brackets, that tells you the positive charge on that transition metal ion.
Sodium Chloride - Na+ Cl- Write the ions with charges
1+ : 1- Write the ratio of the charges
Multiply up if necessary to =0
NaCl Bring together omitting the charges
Copper (II) Chloride Cu2+ Cl- Write the ions with charges
2+ : 1- (x2) Write the ratio of the charges
Cu2+ : Cl-2 Multiply up if necessary to =0
CuCl2 Bring together omitting the charges
Calcium Hydroxide Ca2+ OH- Write the ions with charges
2+ : 1- (x2) Write the ratio of the charges
Ca2+ : (OH-)2 Multiply up if necessary to =0
Ca(OH)2 Bring together omitting the charges
These occur between 2 or more non metals. As neither will donate, they have to share.
Attraction comes between the positive nucleus and the negative electrons that are shared between them.
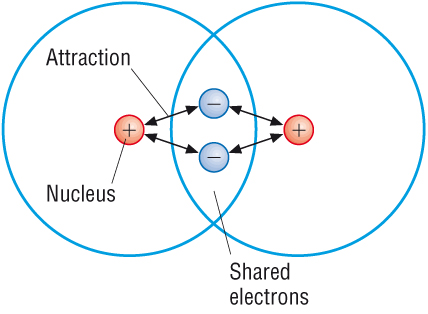
In each case the outer shell has to be filled (reach the noble gas configuration).
Octet rule (more accurately - full outer shell rule)
Single covalent bonds
The single outer shell electrons are involved in a covalent bond.
Each hydrogen has 1 single unpaired electron in its outer shell.
Each hydrogen contributes 1 electron to the covalent bond = 2 electron.
The electrons in a covalent bond are in between the 2 atoms therefore they are directional.
|
Chlorine:
Other examples:
Multiple covalent bonds
|
Each bond is represented by a line:
Questions: 1-2 P55
Dative covalent bonds:
A covalent bond is when each atom provides 1e each to be shared between them.
A dative covalent bond is when both of the bonding electrons are provided by the same atom.
In order for this to happen, an atom or atom in a molecule must have a lone pair of electrons:
The oxonium ion:
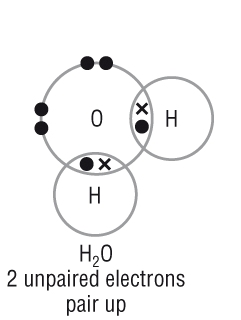 |
|
| HCl(aq) | + | H2O(l) | à | H3O+(aq) | + | Cl-(aq) |
Looking at the atoms for how this molecule is formed:
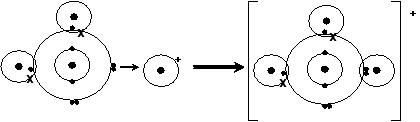
A lone pair of electrons from the water molecule is used to forma dative covalent bond.
The bond is indistinguishable from the other covalent bonds but we use an arrow to show that one of the bonds is dative:
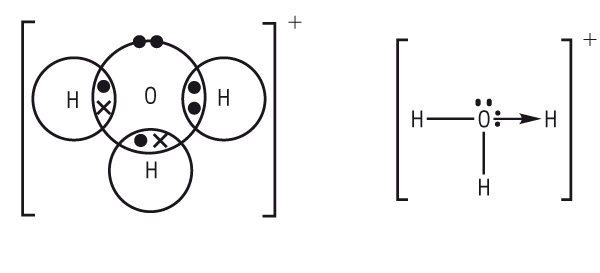
The oxonium ion is responsible for acid reactions and is represented as:
H3O+ or H+(aq)
The ammonium ion:
The same can be done for ammonia. This forms the ammonium ion:
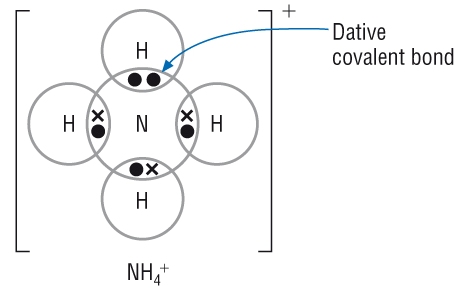 |
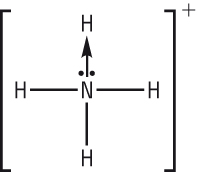 |
Breaking the octet rule
Until now we have used the 'octet' rule to work out how many covalent bonds an atom can make.
 |
|
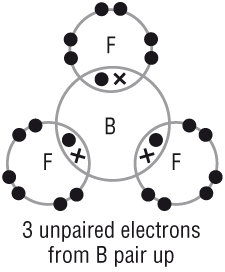
Expanding the octet
Period 3, the electron shell is much larger and can accommodate more covalent bonds.
Some atoms are able to have more than 8 electrons in its outer shell when covalently bonding.
It very much depends on how many of their outer shell electrons are used in bonding / lone pairs:
 |
 |
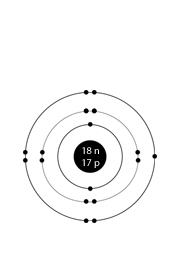 |
| Phosphorous | Sulphur | Chlorine |
| If all unpaired electrons are used then there will be 3 covalent bonds = 6e plus the lone pair of 2e = 8e | If all unpaired electrons are used then there will be 2 covalent bonds = 4e plus the 2 lone pair of 2e = 8e | If all unpaired electrons are used then there will be 1 covalent bond = 2e plus the 3 lone pair of 6e = 8e |
| If the lone pair unpairs then there are 5 unpaired electrons. These will form 5 covalent bonds = 10e | If one of the lone pair unpairs then there are 4 unpaired electrons. These will form 4 covalent bonds = 8e plus the remaining lone pair of 2e = 10e | If one of the lone pair unpairs then there are 3 unpaired electrons. These will form 3 covalent bonds = 6e plus the 2 remaining lone pairs of 4e = 10e |
| If both of the lone pairs unpair then there are 6 unpaired electrons. These will form 6 covalent bonds = 12e | If 2 of the lone pairs unpair then there are 5 unpaired electrons. These will form 5 covalent bonds = 10e plus the remaining lone pair of 2e = 12e | |
| If all of the lone pairs unpair then there are 7 unpaired electrons. These will form 7 covalent bonds = 14e | ||
| Group 5 = 3, 5 bonds | Group 6 = 2, 4, 6 bonds | Group 6 = 1, 3, 5, 7 bonds |
It all depends upon how many electrons are used in bonding.
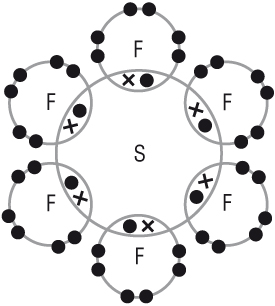 |
|
A better rule:
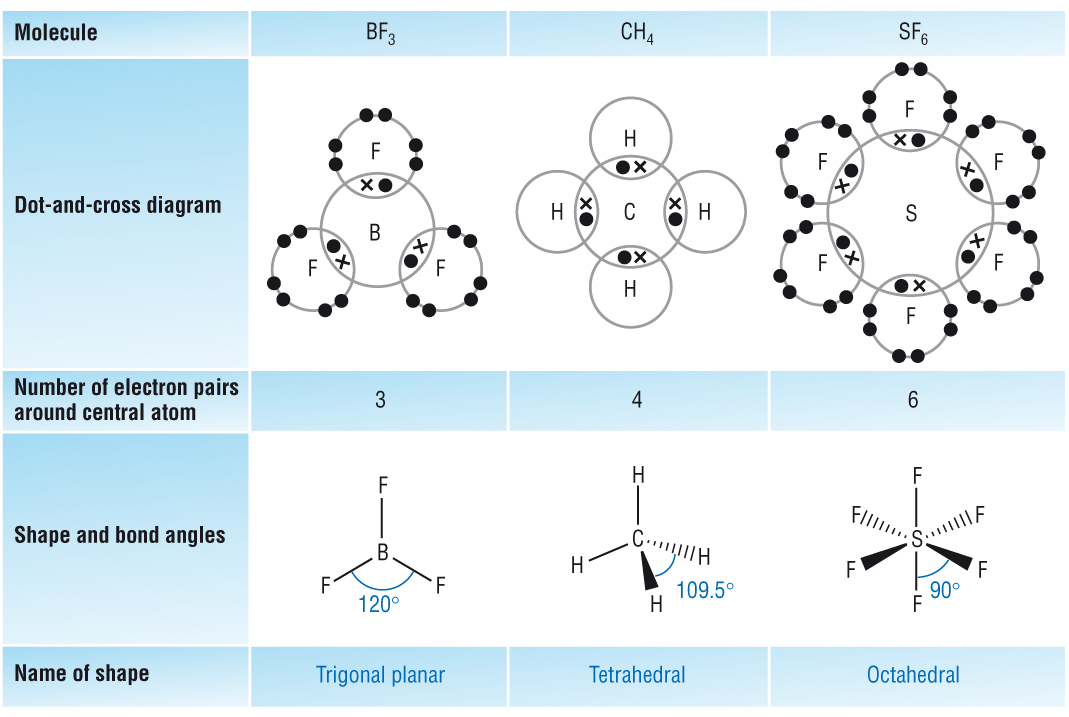
The shape of a molecule is wholly determined by the number of electron pairs (or areas) around a central atom:
Bonding pairs & Lone pairs
Electron pairs repel each other as far as possible.
This repulsion determines the shape and bond angle.
Bonding pairs of electrons
|
|
Trigonal planar |
Lone pair electrons:
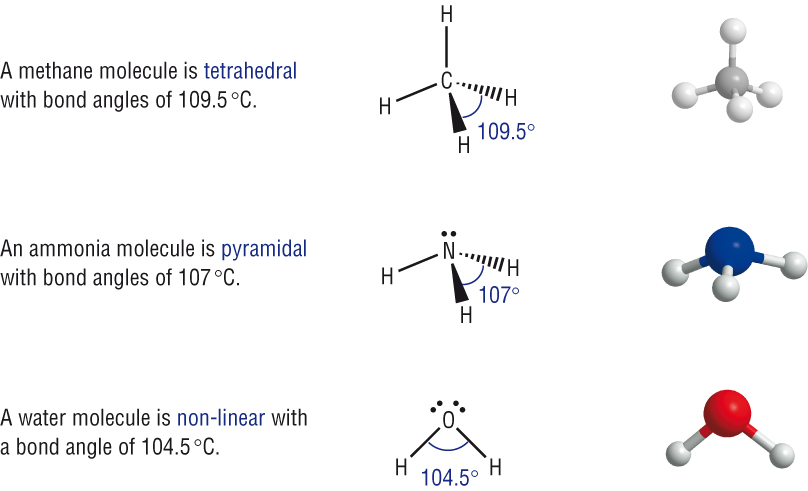 |
Tetrahedral |
| Pyramidal | |
| Non - linear |
Explanation:
Count how many bonding pairs (or areas with double bonds) and lone pair electrons
Pairs of electrons repel other pairs of electrons as far as possible and this determines the shape
Lone pairs are closer to the central atom than bonding pair electrons.
Lone pair electrons will repel other pairs of electrons more than bonding pairs of electrons.
Each lone pair of electrons reduces the bond angle by 2.5o.
Order of repulsion:
Lone Pair – Lone Pair > Lone Pair – Bonding Pair > Bonding Pair – Bonding Pair
 |
 |
Summary:
State the number of bonding and lone pairs of electrons
Pairs of electrons repel as far as possible (lone and bonding)
This determines the shape
Lone pairs repel more than bonding pairs as closer to central atom
Each lone pair reducing the bond angle by 2.5o as it is closer to the central atom
|
3BP (areas) |
4BP |
3BP / 1LP |
2BP / 2LP |
2 Areas (4BP) |
6BP |
|
|
|
|
|
|
|
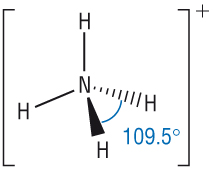
How does the addition of H+ ion change shape of ammonia (water)
|
3BP / 1LP |
|
4BP |
|
|
The addition of a datively bonded H+ ion uses the lone pair converting it to a bonding pair (similar for water) |
|
|
|