|
|
Energy changes of solution:
|
Solute |
Temp start oC |
Temp final oC |
Temp Change oC |
|
|
KCl |
|
|
|
|
|
CaCl2 |
|
|
|
|
|
KNO3 |
|
|
|
|
|
|
||
|
|
|
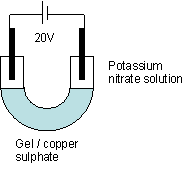
Solution |
Which electrode
does colour go to |
Which ion is
responsible for colour |
Charge on ion |
|
Copper (II) sulphate |
|
|
|
|
Potassium manganate (VII) |
|
|
|