Introduction
Halogenoalkanes have the general formula CnH2n+1X. X is a halogen.
When a hydrogen is replaced by a halogen, the prefix fluoro-, chloro-, bromo- and iodo- are used:-
CH3 Cl Chloromethane
CH3 CH2Br Bromoethane
2 isomers can be derived from a monosubstituted propane:-
CH3 CH2 CH2Cl 1 Chloropropane
CH3 CHCl CH3 2 Chloropropane
Side chains are named in the same way as the alkanes:-
CH3 CH CH2Cl 1 Chloro 2 - methylpropane
CH3
![]()
![]() CH3 CCl CH3 2 Chloro 2 - methylpropane
CH3 CCl CH3 2 Chloro 2 - methylpropane
CH3
Multi halogen substituted compounds use di, tri to indicate how many of that halogen is present in the compound:-
CH2Br CH2Br 1,2 - dibromoethane
SAQ 12.1
The classification of halogenoalkanes
|
|
|
|
|
|
|
|
|
SAQ 12.2
EXPT Reactions of the halogenoalkanes
1) Miscibility with water
To a test tube add about 1cm3 of water and 1cm3 of a halogenoalkane.
Place in a bung and shake well. Record your results.
2) Hydrolysis with sodium hydroxide
To 1cm3 of 20% sodium hydroxide solution in ethanol (CARE).
Add an equal volume of water followed by followed by 0.5cm3 of 2 chloro 2 methylpropane.
Shake from side to side for a minute.
To test for chloride ions add an equal volume of 2M nitric acid to neutralise the sodium hydroxide.
Spot out onto universal indicator paper to ensure the solution is acidic.
Add a few drops of 0.02M silver nitrate. If chloride ions are present a white precipitate of silver chloride will appear.
3) The relative rates of hydrolysis of 1 halogenobutanes.
Arrange 3 test tubes in a row and add 3 drops of halogenoalkane in the order: 1 chlorobutane, 1 bromobutane and 1 iodobutane.
Add 4cm3 of 0.02 silver nitrate to each of the 3 tubes at the same time.
Put all 3 test tubes in a water bath at the same time.
Note the order of precipitation.
Interpretation of the experiments
Physical properties
1) Miscibility with water
Halogenoalkanes have dipole dipole forces of attraction where as water has hydrogen bonding.
The dipole dipole forces of attraction are not similar in strength.
This means that water and halogenoalkanes are immiscible.
They are volatile but not as much as alkanes
SAQ 12.3
Nucleophilic Substitution
2) Hydrolysis with sodium hydroxide
The mechanism:-

Cl is more electronegative than carbon so it has a d-. This means the carbon bonded to the chlorine in the halogenoalkane is Cd+.
It will be an advantage if the attacking group has a negative charge.
Such species are called Nucleophiles and often have negative charges.
This is a substitute reaction as the OH has been substituted for Cl.
As halogenoalkanes do not mix with water, it is mixed with ethanol before adding dilute aqueous sodium hydroxide.
Warming causes nucleophilic substitution and an alcohol is made. A similar but slower reaction occurs with water:
CH3CH2Cl(l) + OH-(aq) ΰ CH3CH2OH(l) + Cl-(aq)
CH3CH2Cl(l) + H2O(l) ΰ CH3CH2OH(l) + HCl(aq)
SAQ 12.4
3) The relative rates of the hydrolysis of 1 halogenobutanes
The rate of precipitation:-
Iodoalkane
Bromoalkane
Chloroalkane
The bond energies give us a good indication of the reactivity of the halogenoalkanes:-
E(C Cl) = 340 KJ mol-1
E(C Br) = 280 KJ mol-1
E(C I) = 240 KJ mol-1
The weakest would be expected to break first.
As this one breaks first it will form a precipitate with Ag+ first:
Ag+(aq) + Cl-(aq) ΰ AgCl(s)
This also tells us that the carbon halogen bond strength overrides the magnitude of the d+ on the carbon.
Electronegativity of the halogens decreases as you go down group 7. This means that the C Cl bond will be more polar than the C Br bond which will be more polar than the C I bond:-
d+ d- d+ d- d+ d-
C Cl > C Br > C I
This means that the carbon atom attached to the chlorine will be the most electron deficient. However the C I bond breaks first.
Reaction with ammonia - You need the mechanism for this reaction
Mixed with ehanolic ammonia and heated under pressure produces amines:
CH3CH2Cl + NH3 ΰ CH3CH2NH2 + HCl
This is a primary amine.
SAQ 12.5
To a round bottom flask add 2cm3 of 20% sodium hydroxide solution in ethanol (CARE).
Add 1cm3 of 1 Chlorohexane
Shake from side to side for a minute, add a few anti bumping granules and reflux gently for 20 minutes.
Allow to cool and set up for distillation. Distill off the liquid +/- 2oC of the boiling point of the product (work it out).
Add a few drops of bromine water bung and shake. Note the results.
Interpretation
When pure alcoholic alkali (KOH) is used and refluxed instead of aq alkali a different reaction occurs:
Mechanism you do not need to know this
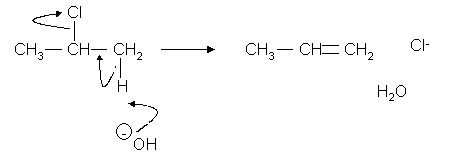
In this reaction the halogen and a hydrogen are both extracted from the molecule.
The OH- is thought to be such a strong base that it pulls a proton off the molecule.
The halogen then separates from the molecule.
This is thought to occur in more than 1 stage.
Summary of the reactions of the halogenoalkanes
1) Preparation halogenoalkanes from Ch11 - Alcohols
Preparation of reagent:-
2NaBr(s) + H2SO4(aq) ΰ 2HBr(aq) + Na2SO4(aq)
Reaction
CH3CH2OH(l) + HBr(aq) ΰ CH3CH2Br(l) + H2O(l)
Mechanism - Protonation, Nucleophilic substitution.
Conditions - Sulphuric acid / potassium halide, heat.
Nucleophile - Br-
2) Rate of hydrolysis then precipitation with silver nitrate, AgNO3
1st Iodo > Bromo > Chloro 3rd
3) Substitution by a aq hydroxyl group, OH-
Reaction
CH3CH2Cl(l) + OH-(aq) ΰ CH3CH2OH(l) + Cl-(aq)
Mechanism - Nucleophilic substitution.
Conditions - aq potassium hydroxide, KOH.
Nucleophile - OH-
4) Substitution by an amine group, NH3
Reaction
CH3CH2Cl(l) + NH3(aq) ΰ CH3CH2NH2(l) + Cl-(aq)
Mechanism - Nucleophilic substitution.
Conditions - Alcoholic (ethanol) ammonia, NH3 / heat / Pressure.
Nucleophile - NH3
5) Elimination to an alkene
Reaction
CH3CH2Cl(l) + OH-(aq) ΰ CH2 = CH2(l) + Cl-(aq) + H2O(l)
Mechanism - Elimination.
Conditions - alcoholic (ethanol) potassium hydroxide, KOH / reflux with heat.
Used in routes for synthesis
This makes them more useful than the halogenoalkane itself.
Ibuprofen requires joining an alkyl group to benzene. This is done using a halogenoalkane. Ibuprofen is used as an anti inflammatory for eg arthritis.
Direct applications
PVC poly(chloroethane) used in double glazing.
PTFE poly(tetrafluoroethane) used in the non stick coating on saucepans and in waterproof clothes.
CFCs ChloroFluoroCarbons used as coolants in refrigerators, aerosol propellants and blowing agents. Also used as a dry cleaning solvent or degreasing agent for circuit boards.
BCF Bromochlorodifluoromethane used in firefighting. When some combustible materials are ignited free radicals are produced which propagate the reaction forming more free radicals. Terminating these free radicals with BCF can extinguish the reaction. It is the bromine that is responsible: At high temperatures a bromide radical is formed which mops up other free radical produced. This quenches the reaction.
Trouble with the ozone layer
CFCs absorb more IR radiation than CO2 but they have little effect due to their low abundance.
CFCs have a more devastating effect on the ozone layer.
The ozone layer filters out harmful UV light which can cause skin cancer.
CFCs were used in refrigeration and aerosol propellants.
CFCs were used as they liquefy easily when compressed and are unreactive, non flammable and non toxic.
Before CFCs ammonia or sulphur dioxide was used. In the 1920s these caused a number of deaths.
The stability of CFCs has been the problem and the concentration has slowly built up in the atmosphere.
In the stratosphere CFCs absorb UV light causing photodissociation of the C Cl bond:
CF2Cl2 ΰ CF2Cl. + Cl.
It is the very reactive chlorine radical that catalyses the decomposition of ozone to oxygen:
3O2 ΰ 2O3
This is an example of chemists responding to the needs of society.
Ammonia and sulphur dioxide was replaced with CFCs due to their toxicity with no apparent effect on the environment.
Years later we can now see the effect of CFCs in the atmosphere.
Scientists are now working on an ozone friendly compound. 1,1,1,2 tetrafluoroethane, CF3CH2F is now being used.
The hydrogens increases its reactivity which means it is broken down lower in the atmosphere.
Any of these compounds that get into the stratosphere do not decompose producing chlorine radicals.