The greenhouse effect - global warming:
 |
- Radiation from the sun reaches the planet.
- The radiation is absorbed by the Earth and re
emitted as IR radiation.
- Most of this IR radiation goes back into
space but some is absorbed by gases in the atmosphere.
- These gas molecules absorb the IR radiation
then re emit it as energy, this energy warms up the atmosphere.
- These gases are: water, methane and
carbon dioxide.
|
How do gases absorb radiation?
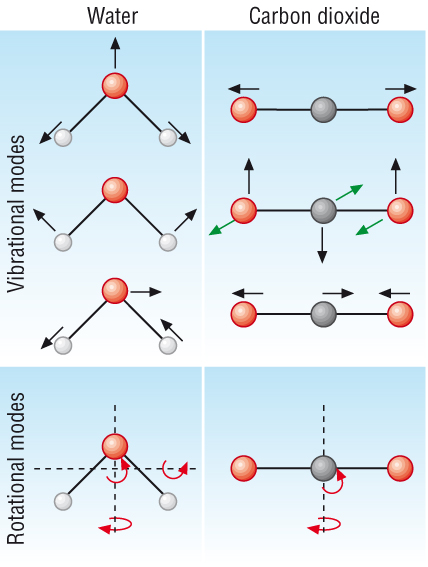 |
- Just like IR spectroscopy, the bonds in these
greenhouse gases absorb IR radiation in their bonds.
- The bonds vibrate absorbing the IR radiation.
- Different gases will absorb different amounts
of IR radiation.
- 3 factors determine the impact a gas has on
Global warming:
- Its concentration in the atmosphere
- Its ability to absorb IR radiation
- Its lifetime in the atmosphere
- These 3 factors make up the GWP (Global
Warming Potential)
- The term Climate Change explains that
although the average temperature of the planet is rising, different
areas around the planet will suffer from extreme weather patterns.
|
Solutions to the Greenhouse Effect:
- Obviously alternative fuels such as: Wind,
tidal, solar, nuclear. But we still need fossil fuels to meet the
energy demands of the planet.
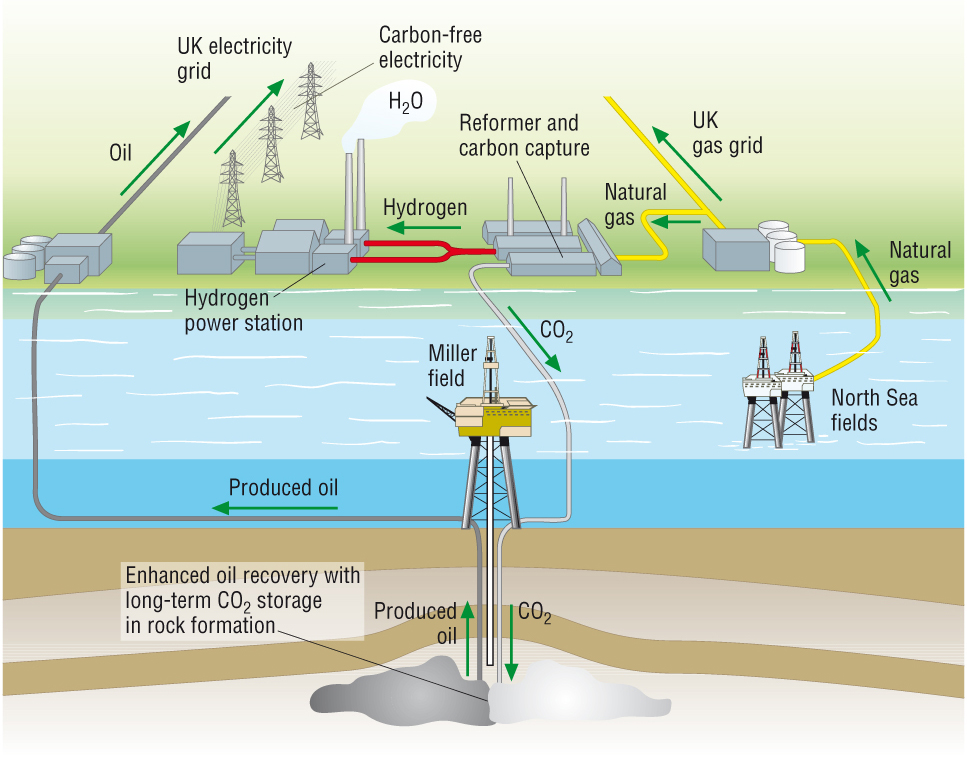
Carbon Capture and Storage, CCS:
| |
CH4(g) |
+ |
2O2(g) |
à |
CO2(g) |
+ |
2H2O(g) |
|
|
|
|
- Obviously this produces
CO2 which is emitted into the
atmosphere.
- The fuel can be decarbonised:
| |
CH4(g) |
+ |
2H2O(g) |
à |
CO2(g) |
+ |
4H2(g) |
|
|
|
|
- The CO2
can be separated and pumped into oil wells to get the last bit of oil out.
- H2 is produced which is a clean
fuel as it only produces water vapour.
- The CO2
is now trapped in the oil well and not emitted into the atmosphere:
Storage as carbonates:
- Mineral storage aims to store the CO2
locked up in minerals as carbonates, CO3:
| |
CaO(s) |
+ |
CO2(g) |
à |
CaCO3(s) |
|
|
|
|
|
|
| |
MgO(s) |
+ |
CO2(g) |
à |
MgCO3(s) |
|
|
|
|
|
|
- This process occurs naturally but is very slow,
More research is needed if this is to be a viable storage option.
The ozone layer
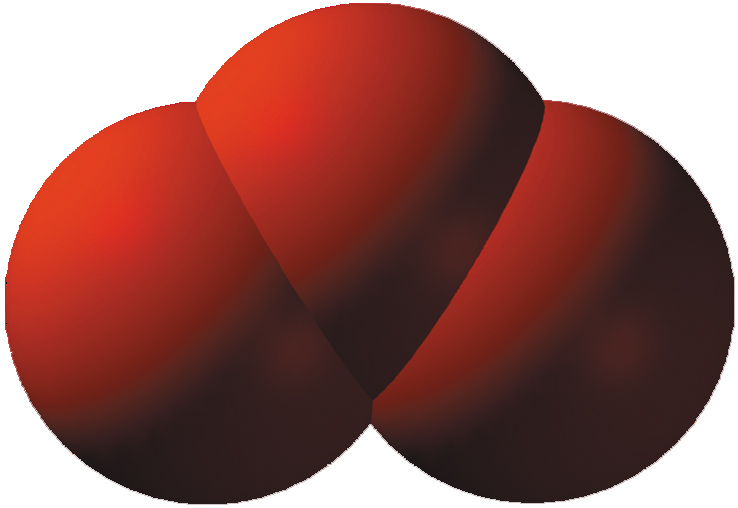 |
- Ozone is 3 oxygen atoms joined together.
|
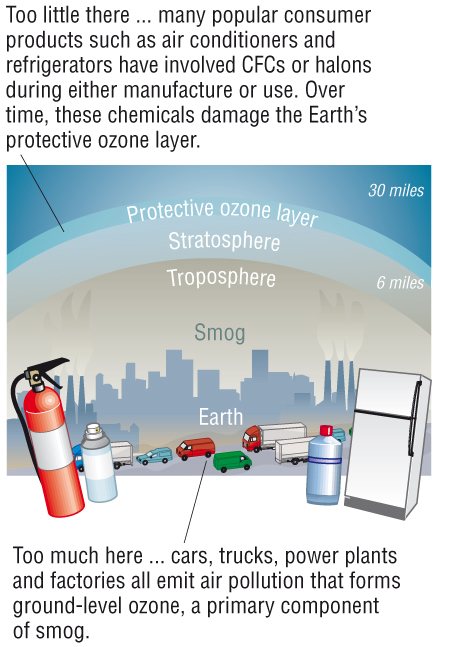 |
- We are producing ozone where we don't want it
and destroying it where we do want it.
- Low level ozone in the troposphere causes
respiratory problems.
- The destruction of high level ozone allows
harmful UV radiation to reach earth.
|
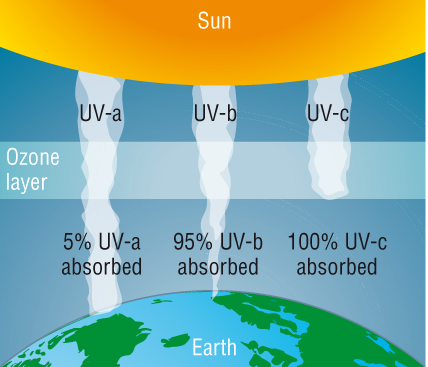 |
- Ozone acts like a big pair of sunglasses
filtering out most of the harmful UV radiation.
- Prior to the formation of ozone our planet
was scorched and no life could survive.
- UV radiation is divided into a, b, and c.
- C is the most harmful and is blocked out
completely by ozone.
- A is the weakest and only a small amount is
absorbed by ozone. This is the one that gives you a tan and
ages your skin prematurely.
|
Ozone formation:
- The first step is the homolytic fission of an oxygen
molecule by UV light:
- This is an oxygen atom which contains 2 unpaired
electrons, sometimes called a di - radical.
- The oxygen atoms reacts with oxygen molecules forming
ozone. This gives out heat - exothermic:
| |
O(g) |
+ |
O2(g) |
à |
O3(g) |
+ |
Heat |
|
|
|
|
How the ozone layer works:
- Ozone absorbs UV radiation breaking the molecule into
oxygen molecules and atoms:
| |
O3(g) |
+ |
UV |
à |
O2(g) |
+ |
O(g) |
|
|
|
|
- The oxygen atom then react with an oxygen molecule:
| |
O(g) |
+ |
O2(g) |
à |
O3(g) |
+ |
Heat |
|
|
|
|
- Overall, UV is converted to heat energy and this
process continues until the 2 reactions reach an equilibrium:
Removal of ozone:
- Oxygen atoms remove ozone. This is a slow
reaction but the balance can be affected easily (later).
Ozone depletion
-
Free radicals react fast and the chlorine radical could
decompose as many as 100000 ozone molecules.
-
The oxygen radical in step 2 is produced from UV
dissociation of oxygen and ozone in the stratosphere.
2) Nitrogen oxide:
- Nitrogen oxides are formed by lightening strikes and
aircraft engines:
Controlling air
pollution
The internal combustion engine:
- The high pressures and temperatures causes many
atmospheric pollutants:
1) Carbon monoxide:
- Is poisonous, combines with haemoglobin in place of
oxygen.
- Reduces the ability to perform complex tasks,
dexterity,vision
2) Nitrogen oxides (NOx):
- The high temperatures in a car engine will break the
triple bond in nitrogen, N2 allowing them to react with oxygen
forming nitrogen oxides.
- 2 main oxides are nitrogen monoxide, NO and nitrogen
dioxide, NO2.
- Produces low level ozone and nitric acid (acid rain)
- NOx irritate the respiratory system
(asthmatics).
3) Unburnt hydrocarbons:
- Volatile organic compounds (VOC's) are unburnt fuels
released in exhaust gases.
- Benzene compounds are carcinogenic and of particular
concern.
- These benzene compounds will react with NO2
causing low level ozone.
- Low level ozone irritates the respiratory system.
The catalytic converter:
- These are made from Pt Rh Pd metals in a
honeycombed structure to increase surface area forming the catalyst.
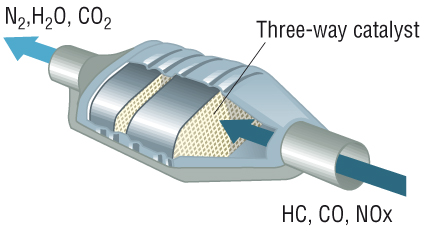
- There are 2 types of catalytic converters:
1) Oxidation catalytic converters - diesel
engines:
- Convert CO to CO2 and oxidise VOC's:
| |
C12H26(l) |
+ |
18.5O2(g) |
à |
12CO2(g) |
+ |
13H2O(g) |
|
|
|
|
- A complex filter also removes any particulate matter.
2) 3-way catalyst - petrol engines:
- As above but also converts removes NO:
| |
2NO(g) |
+ |
2CO(g) |
à |
N2(g) |
+ |
2CO2(g) |
|
|
|
|
How the catalyst functions:
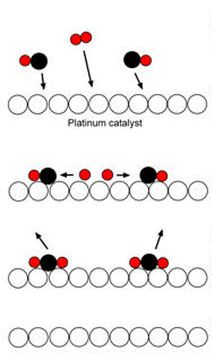 |
Adsorption: As the molecules diffuse
over the surface of the catalyst some of the molecules are held on the
metal surface. |
| Reaction: Temporary bonds are formed
between the molecules and the surface of the catalyst. This
weakens the bonds in the molecules. The molecules can now react. |
| Desorption: After the reaction the
products are desorbed from the catalyst and diffuse away. |
Green chemistry
Sustainability and the green chemist
The 12 principles:
1) Prevention
2) Atom economy
3) Less hazardous chemical synthesis
4) Design safer chemicals
5) Safer solvents and auxiliaries
6) Design for energy efficiency
7) Use of renewable feedstocks
8) Reduce derivatives
9) Catalysts
10) Design for degradation
11) Real time analysis for pollution prevention
12) Inherently safer chemistry for accident prevention
CO2 -
villain to saviour
Using CO2
- CO2 can be used instead of allowed to
pollute the atmosphere.
- It can be collected from fermentation or the
decarbonisation of methane
1) In foam:
- It can be used in expanded foam instead of CFC's
2) As a solvent:
- By altering temperature and pressure CO2
can be converted to a liquid (known as a super critical fluid, scCO2)
- An advantage is that it is not flammable or toxic.
a) Decaffeinating coffee:
- It has the advantage that it removes 97 - 99% without
affecting the taste:
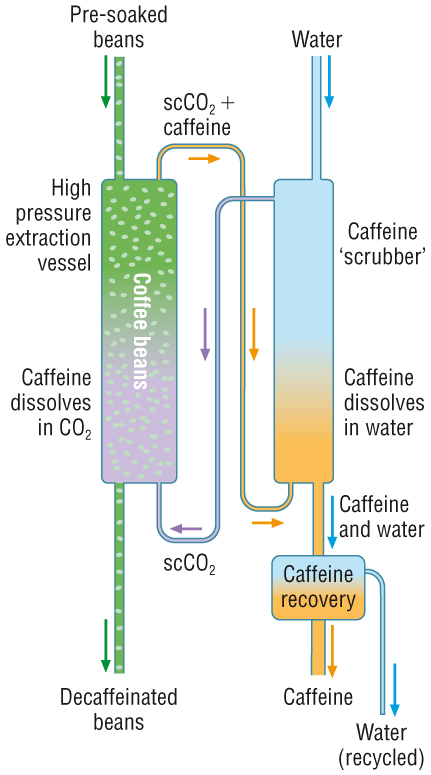
b) Extracting beer flavour:
- As with decaffeinating coffee, scCO2 can
remove the beer flavour from hops without losing flavour or increasing
toxicity risks.
c) Dry cleaning:
- scCO2 has now replaced C2Cl4
and CCl4, known carcinogens.
- It has the same properties for dissolving greases and
oils but without the risks.
d) Toxic waste treatment:
- scCO2 can remove dissolved organic
compounds (toxic waste) from waste mixtures.
e) For chemical synthesis:
- scCO2 ability as a solvent can be
controlled by varying temperature and pressure.
- This allows you to produce the desired product with
fewer co products.
- It also makes separation much easier.