| Collision theory | |
| Effect of temperature | |
| Effect of concentration | |
| Effect of pressure | |
| Activation energy | |
| Catalysis |
Rate of reaction
The rate of a reaction is defined as the change in concentration of a reactant or product in a given time
| Rate | = | Change in concentration | Units: | mole dm-3 | = | mol dm-3 s-1 | |||
| Time | s |
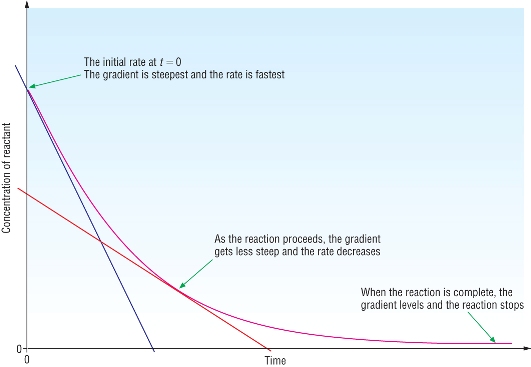
Representing concentration
Measuring rates
| SLOWS DOWN | As fewer collisions take place | |
| STOPS | When one reactant has been used up |
| y/x | Concentration / time | |
| Gradient | Rate |
A) Measuring the decrease in concentration of a reactant:

A) Measuring the increase in concentration of a product:

Qu 1 - 3 P113
Obtaining data for a concentration - time graph:
| Acid - Base reactions | Gas production | Visible changes |
| Titrations |
Change in volume (gas collection) |
Precipitation (colorimeter) |
| pH meter | Loss in mass | Colour changes (colorimeter) |
Using a colorimeter
| Fe(s) | + | CuSO4(aq) | à | Fe2SO4(aq) | + | Cu(s) | |
| Blue solution | Colourless solution |

| SO2Cl2(g) | à | SO2(g) | + | Cl2(g) |
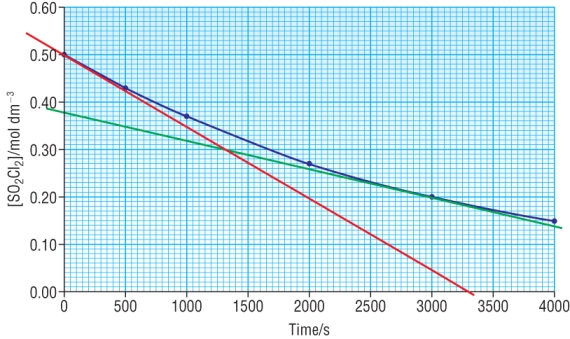 |
Red tangent is at t = 0 (initial rate): rate = 0.50 - 0.00 = 1.5 x 10-4 mol dm3 s-1 3300 - 0 |
|
Green tangent is at t = 3000: rate = 0.38 - 0.14 = 6.0 x 10-5 mol dm3 s-1 4000 - 0 |
Question 1 P 115
Orders:
| 1) No effect | |
| 2) Double the rate | |
| 3) Quadruple the rate |
|
Rate is proportional to the concentration |
|||
| Rate | a |
[A] x |
|
| x |
is the order |
||
The rate equation and the rate constant:
|
Rate is proportional to the concentration |
||||
| Rate | = | k |
[A] x |
|
To look at how the order effects the rate we'll keep the numbers simple.
Lets make:
|
k = 1 |
||||
| [A] | = |
1 |
Doubling the concentration of A from 1M to 2M | |
| [A] | = |
2 |
Zero order - where x = 0:
| Rate | = | k |
[A] x |
||
| Rate | = | 1 |
[1] 0 |
Rate = 1 | |
|
Now double the concentration of A |
|||||
| Rate | = | 1 | [2] 0 |
Rate = 1 |
|
Doubling the concentration has no effect on the rate
First order - where x = 1:
| Rate | = | k |
[A] x |
||
| Rate | = | 1 |
[1] 1 |
Rate = 1 | |
|
Now double the concentration of A |
|||||
| Rate | = | 1 | [2] 1 |
Rate = 2 |
|
Doubling the concentration doubles the rate wrt [A]
Second order - where x = 2:
| Rate | = | k |
[A] x |
||
| Rate | = | 1 |
[1] 2 |
Rate = 1 | |
|
Now double the concentration of A |
|||||
| Rate | = | 1 | [2] 2 |
Rate = 4 |
|
Doubling the concentration quadruples the rate wrt [A]
The rate equation and the rate constant:
|
Rate is proportional to the concentration |
||||
| Rate | = | k |
[A] x |
|
Remember - a reaction usually has more than one reactant:
| A | + | B | + | C | à | Products |
| Rate | a |
[A] 0 |
Rate | a |
[B] 1 |
Rate | a |
[C] 2 |
Combining these 3 give:
| Rate | a |
[A] 0 |
[B] 1 | [C] 2 |
| Rate | = | k |
[A] 0 |
[B] 1 | [C] 2 |
| Rate | = | k | [B] 1 | [C] 2 |
Overall order:
| Rate | = | k | [B] 1 | [C] 2 |
Orders of reaction and the rate equation can only be determined experimentally.
Units of rate constants:
| Rate | = | k [A] 1 | Rate | = | k [A] 2 | Rate | = | k [A] [B] | |||||
| k | = | Rate | k | = | Rate | k | = | Rate | |||||
| [A] 1 | [A] 2 | [A] [B] | |||||||||||
| k | = | mol dm-3 s-1 | k | = | mol dm-3 s-1 | k | = | mol dm-3 s-1 | |||||
| mol dm-3 | mol dm-3 | mol dm-3 | mol dm-3 | mol dm-3 | |||||||||
| k | = |
|
k | = |
|
k | = |
|
|||||
|
|
|
mol dm-3 |
|
mol dm-3 | |||||||||
| k | = | s-1 | k | = | s-1 | k | = | s-1 | |||||
| mol dm-3 | mol dm-3 | ||||||||||||
| k | = | dm3 mol-1 s-1 | k | = | dm3 mol-1 s-1 | ||||||||
Questions 1 - 3 P117
![]() and / or
carry out method 2 at home
and / or
carry out method 2 at home
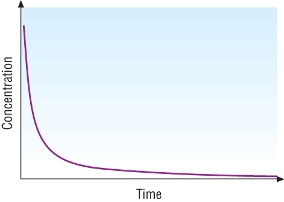
Concentration - time graphs:
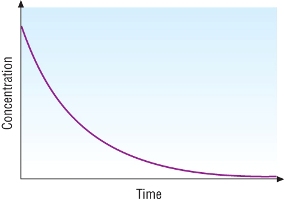
Half - life:
Concentration - time graph for a first order reaction:
| 2N2O(g) | à | 2N2(g) | + | O2(g) |

Other concentration - time graphs:
| Zero order | First order | Second order |
 |
 |
 |
|
|
|
Qu 1 - 2 P 119
Qu 1 P159
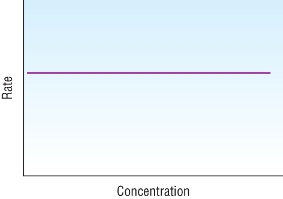
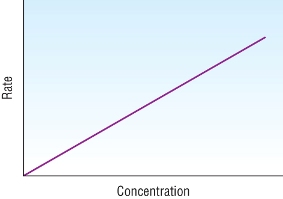
Orders from rate - concentration graphs
| Zero order | First order | Second order |
 |
 |
 |
Horizontal straight line |
Proportional straight line |
Proportional curve |
Initial rates:
A) From concentration - time graphs:
B) From clock reactions:
| appearance of a precipitate | |
| disappearance of a solid | |
| a change in colour |
Example - Sodium thiosulphate and hydrochloric acid:
| Na2S2O3(aq) | + | 2HCl(aq) | à | 2NaCl(aq) | + | S(s) | + | SO2(aq) | + | H2O(l) |
|
Na2S2O3 |
HCl |
|
 |
 |
|
Proportional straight line 1st order wrt [Na2S2O3] |
Horizontal straight line zero order wrt [HCl] |
| Rate | = | k [Na2S2O3]1 [HCl]0 |
| Rate | = | k [Na2S2O3] |
Questions 1,2 P 121
Initial rates and the rate constant
Determination of orders by inspection:
Example:
Consider the reaction:
|
2NO(g) |
+ | O2(g) | à | 4NO2 |
| Experiment | Initial concentration [NO] (mol dm-3) | Initial concentration [O2] (mol dm-3) | Initial rate (mol dm-3 s-1) |
| 1 | 0.00100 | 0.00100 | 1.82 x 10-6 |
| 2 | 0.00100 | 0.00300 | 5.46 x 10-6 |
| 3 | 0.00200 | 0.00100 | 7.28 x 10-6 |
Order for NO:
Order for O2:
| Rate | = | k [NO]2 [O2]1 |
| Rate | = | k [NO]2 [O2]1 | Rate | = | k [NO]2 [O2]1 | |||
| k | = | Rate | k | = | Rate | |||
| [NO]2 [O2]1 | [NO]2 [O2]1 | |||||||
| k | = | 5.46 x 10-6 | k | = | mol dm-3 s-1 | |||
|
(0.00100)2
x (0.00300)
|
(mol dm-3)2 | mol dm-3 | ||||||
| k | = | 1820 | k | = |
|
|||
| (mol dm-3)2 |
|
|||||||
| k | = | s-1 | ||||||
| (mol dm-3)2 | ||||||||
| k | = | dm6 mol-2 s-1 | ||||||
The rate constant, k:
| Concentration | |
| rate constant |
The effect of temperature on the rate constant

Questions 1-2 P123
| Step 1 | Step 2 | Step 3 | ||
| Get out 2 slices of bread | Butter the bread add the ham between the slices and assemble the sandwich | Put the sandwich in a sandwich bag |
| NO2(g) | + | CO(g) | à | NO(g) | + | CO2(g) |
| Second order wrt NO2(g) | |
| Zero order wrt CO(g) |
|
First step |
NO2 | + | NO2 | à | Slow - Rate Determining Step |
| First step | NO2 | + | NO2 | à | Slow - Rate Determining Step | |||
| Second step | + | CO | à | + | CO2 | Fast | ||
| Overall | NO2 | + | CO | à | NO | + | CO2 |
| First step | NO2 | + | NO2 | à | Slow - Rate Determining Step | |||
| Second step | + | CO | à | NO2 | + | CO2 | Fast | |
| Overall | NO2 | + | CO | à | NO | + | CO2 |
| First step | NO2 | + | NO2 | à | NO3 | + | Slow - Rate Determining Step | |
| Second step | NO3 | + | CO | à | NO2 | + | CO2 | Fast |
| Overall | NO2 | + | CO | à | NO | + | CO2 |
| First step | NO2 | + | NO2 | à | NO3 | + | NO | Slow - Rate Determining Step |
| Second step | NO3 | + | CO | à | NO2 | + | CO2 | Fast |
| Overall | NO2 | + | CO | à | NO | + | CO2 |
Summary:
If a reactant appears in the rate equation, then that reactant takes part in the slow step of the reaction (RDS). If it does not appear in the rate equation then the reactant does not participate in the slow step (RDS).