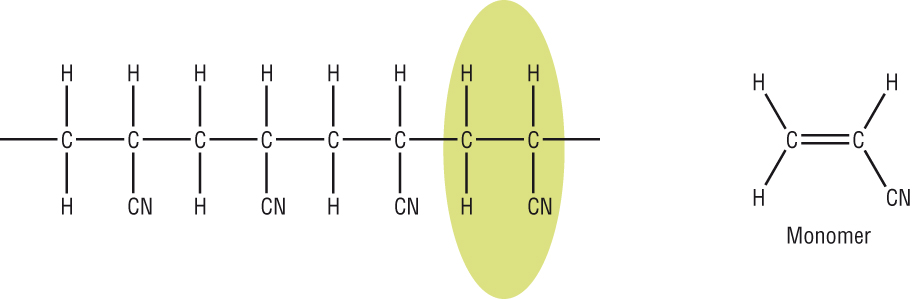
-
Hydrocarbons contain only the elements hydrogen and carbon.
-
Found as fossil deposits of crude oil and natural gas.
-
They are made from naturally decaying plants and animals over millions of years
-
Crude oil is a mixture of around 150 hydrocarbons which are mainly straight chain alkanes.
-
Each source varies in its mixture slightly.
-
Crude oil does not ignite easily. Only when separated into its constituent components is it valuable
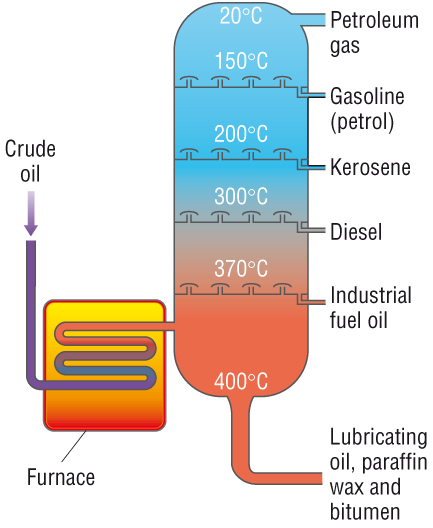
Fractional distillation of crude oil:
-
Crude oil is a mixture of different hydrocarbons with a large range of boiling points.
-
Fractional distillation is used to separate crude oil into fractions.
-
Each fraction is made up of many different hydrocarbons but with more similar boiling points.
-
Further distillation of these fractions can occur when a desired hydrocarbon is needed.
 |
|
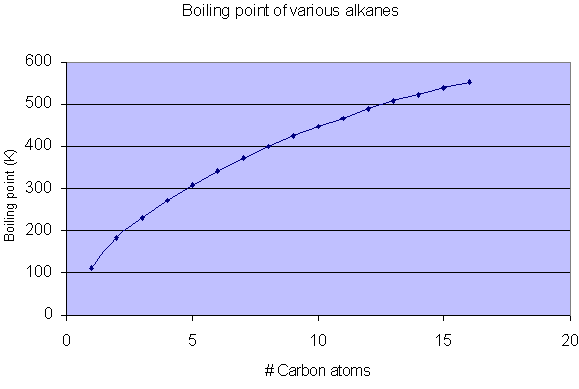
Boiling points of the alkanes:
 |
-
The graph shows - the larger the carbon chain, the higher the boiling point:
-
Weak Van der Waal's forces of attraction exist between the alkanes.
-
The more carbons and hydrogen's there are, the more electrons there are in the molecule.
-
The more electrons there are in the molecule, the stronger the Van der Waal's forces of attraction.
-
This means that as the carbon chain increases, so does the strength of the Van der Waals forces of attraction.
-
This means that more energy is needed to overcome these attractive forces.
| Pentane |
|
309K |
| 2-methylbutane |
|
301K |
| 2,2-dimethylpropane |
|
283K |
-
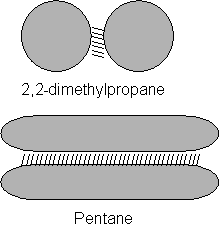
Each isomer has the same number of electrons but the strength of these forces are clearly not the same. This is indicated by the different boiling points.
-
The shape of the molecule must have an effect on the boiling points. The more branches the lower the boiling point which means weaker Van Der Waals.
-
The long shape of pentane allows close packing and maximum surface area for Van Der Waals. This gives optimum interactions
-
More branches means the molecules can’t pack as closely.
-
It also means that the surface area for Van Der Waals is reduced, therefore the energy needed to overcome Van der Waals forces is reduced (lower boiling point)
 |
|
Questions 1-2 P119
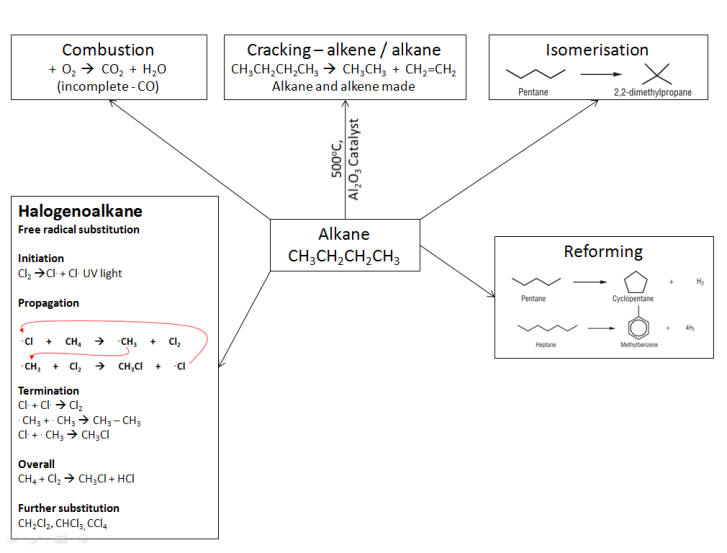
The combustion of alkanes:
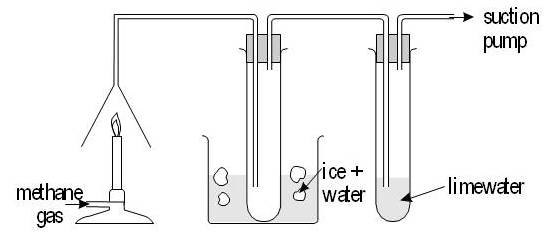
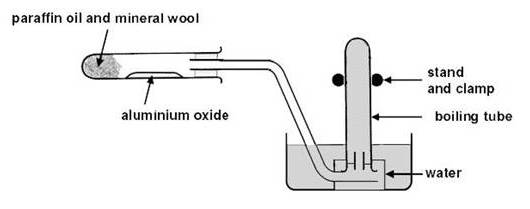
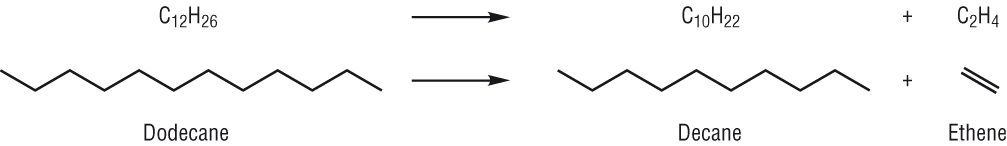
Cracking:
|
|
|
 |
Producing branched chains:
-
Straight chain alkanes have a tendency to pre ignite in a combustion engine whereas branched chains do not.
-
Branched chain alkanes burn cleaner than straight chain alkanes so they are usually converted using:
-
Isomerisation reactions:
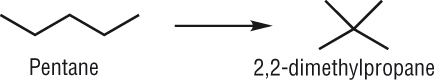 |
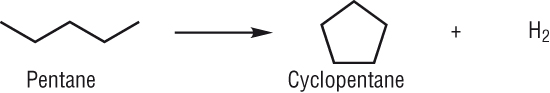
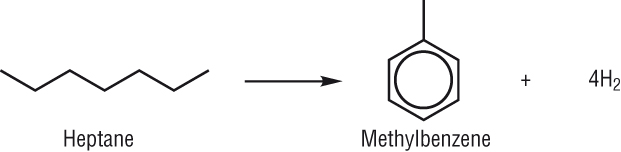
Producing cyclic hydrocarbons:
-
Again as straight chain alkanes have a tendency to pre ignite they are also converted to cyclic and aromatic hydrocarbons.
-
Any hydrogen produced is used in other processes - ammonia production, margarines.
-
These also burn cleaner than straight chain alkanes so they are usually converted using:
-
Reforming reactions:
 |
 |
Improving fuels:
- Fuels have an octane rating (Research Octane Rating, or RON) which rates how well a fuel burns:
| 100% | Burns efficiently |
| 0% | Burns inefficiently |
-
Branched and cyclic alkanes burn much more efficiently than straight chain alkanes hence the conversions (above).
Questions 1-4 P121
Questions 6-9 P143
Fossil fuels and fuels of the future
The crude oil economy:
-
90% of all crude oil is used as a source of fuels to generate electricity or used for transport.
-
Plastics, pharmaceuticals, cosmetics etc are also sourced from crude oil.
-
Our reliance on crude oil is worrying as oil deposits are running out!
The use of crude oil for fuels:
-
The majority of crude oil is the alkanes - chains and branches. These are good fuels.
-
As the availability of oil decreases the price increases.
-
Crude oil as a fuel contributes to atmospheric pollutions:
| Carbon monoxide | - toxic gas | |
| Carbon dioxide | - global warming | |
| Nitrogen oxides | - acid rain / forest destruction | |
| Sulphur oxides | - acid rain |
-
All of these reasons has precipitated a shift away from the use of fossil fuels
The greenhouse effect - global warming:
 |
|
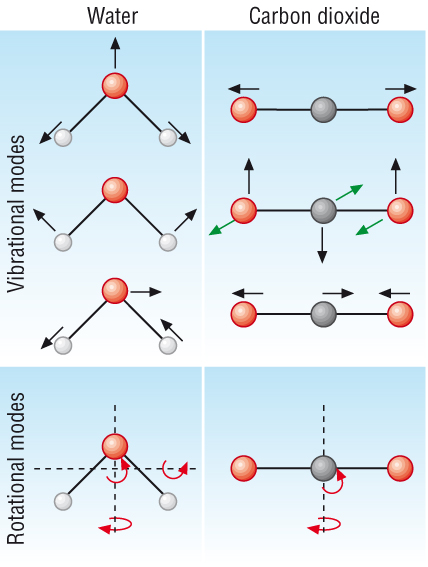
How do gases absorb radiation?
 |
|
Solutions to the Greenhouse Effect:
- Obviously alternative fuels such as: Wind, tidal, solar, nuclear. But we still need fossil fuels to meet the energy demands of the planet.
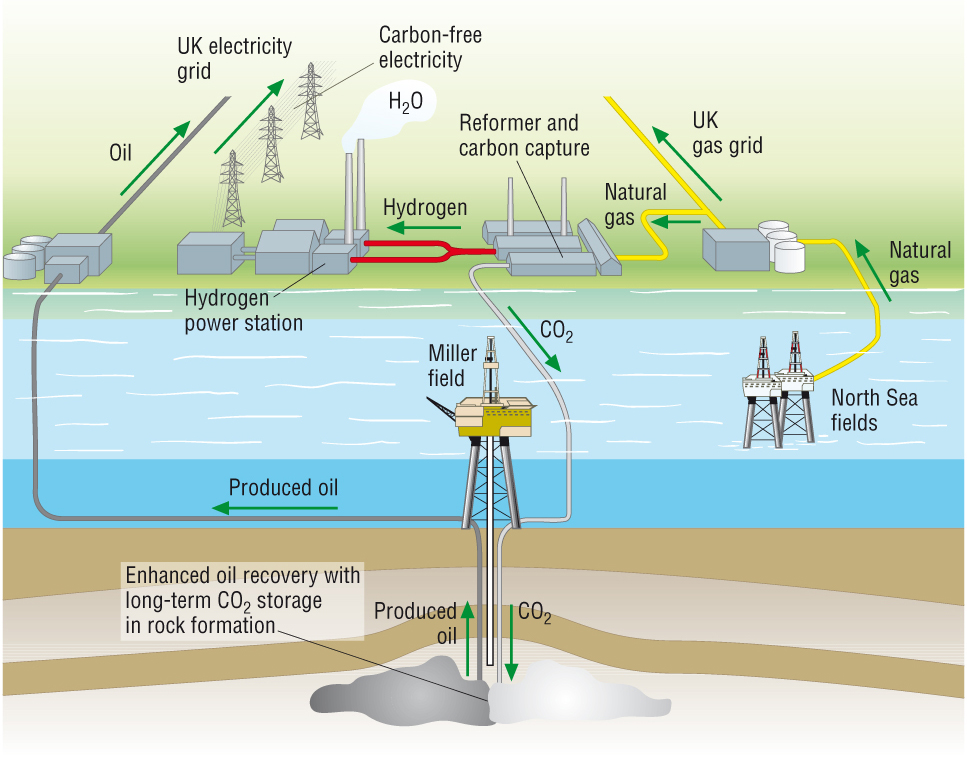
Carbon Capture and Storage, CCS:
- When methane burns:
| CH4(g) | + | 2O2(g) | à | CO2(g) | + | 2H2O(g) |
- Obviously this produces CO2 which is emitted into the atmosphere.
- The fuel can be decarbonised:
| CH4(g) | + | 2H2O(g) | à | CO2(g) | + | 4H2(g) |
- The CO2 can be separated and pumped into oil wells to get the last bit of oil out.
- H2 is produced which is a clean fuel as it only produces water vapour.
- The CO2 is now trapped in the oil well and not emitted into the atmosphere:
 |
Storage as carbonates:
- Mineral storage aims to store the CO2 locked up in minerals as carbonates, CO3:
| CaO(s) | + | CO2(g) | à | CaCO3(s) |
| MgO(s) | + | CO2(g) | à | MgCO3(s) |
- This process occurs naturally but is very slow, More research is needed if this is to be a viable storage option.
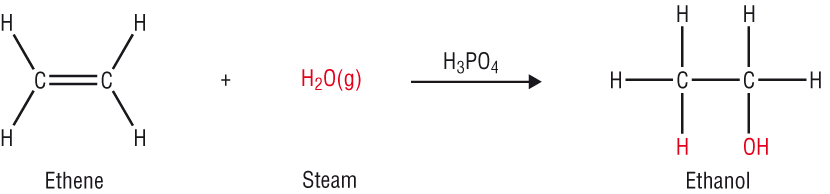
Fuels of the future:
-
Biofuel is made from the fermentation of plant material such as rape or sugar cane to produce ethanol.
-
The idea is that as the plant grows it absorbs CO2. When the ethanol is used as a fuel that CO2 is released. This makes it 'carbon neutral'.
-
The ethanol can be used as a fuel or added to petrol to make petrol burn more efficiently.
-
Biodiesel is made from rapeseed. It can be used pure in an diesel engine but is more commonly added to normal diesel.
Questions P123 Qu 1 - 3
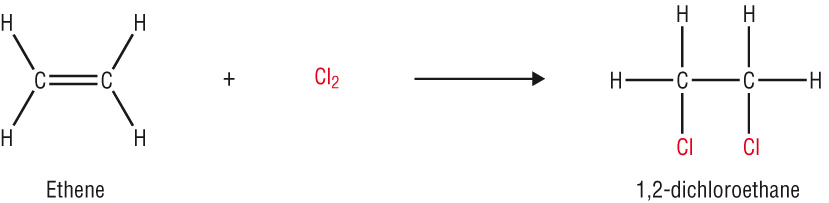
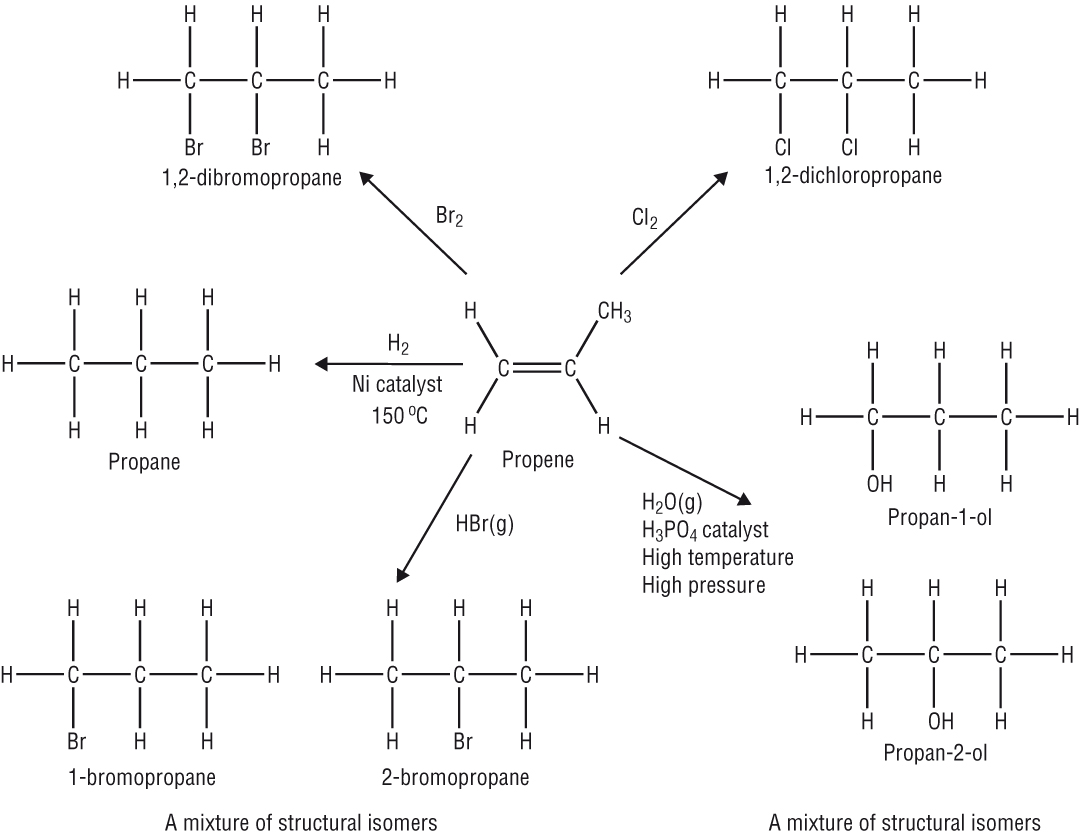
Substitution reactions of the alkanes
Halogenation of the alkanes:
Overall
Further substitution CH2Cl2, CHCl3, CCl4
 |
-
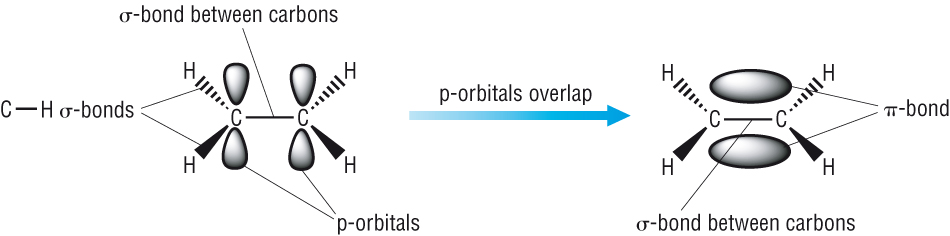
The double bond consists of a sigma bond (s) and a pi bond (p).
-
The p bond consists of 2 lobes one on each side of the sigma bond.
-
The 2 lobes overlap to produce a p bond.
-
To ensure maximum overlap, ethane must be a planar molecule.
-
The asymmetric shape of the 2 bonds locks the molecule around the double bond
-
This means that there is no free rotation about these bonds.
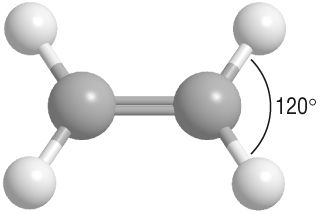
-
The p bond changes the shape around the carbon atom to a trigonal planar with a bond angle of 120o:
Cyclic alkenes:
 |
|
 |
|
Questions P127 1-4
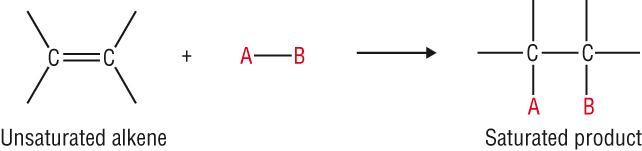
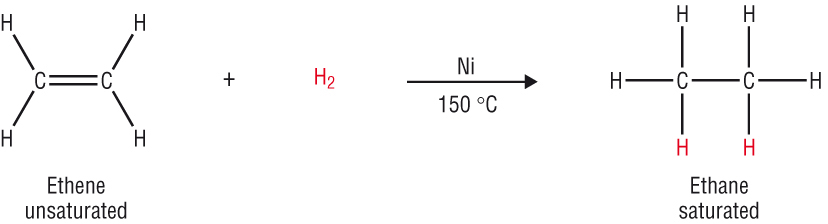
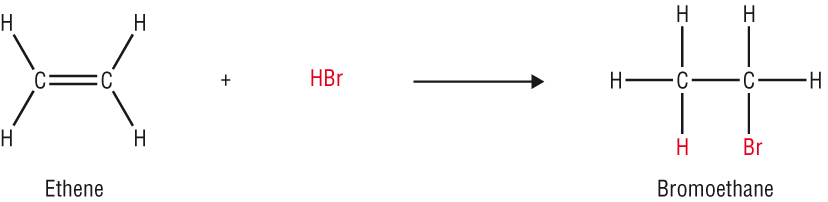
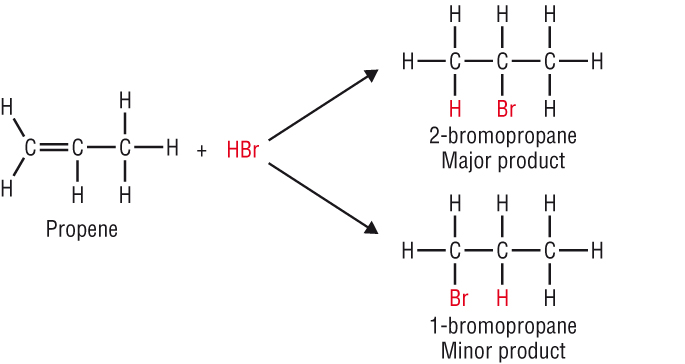
The reactivity of the C=C double bond:
-
The extra electrons in the C=C makes it more reactive as it is more electron rich.
-
The relative strengths of the 2 bonds are:
| Bond | Bond enthalpy Kj mol-1 |
| C-C | +347 |
| C=C | +265 |
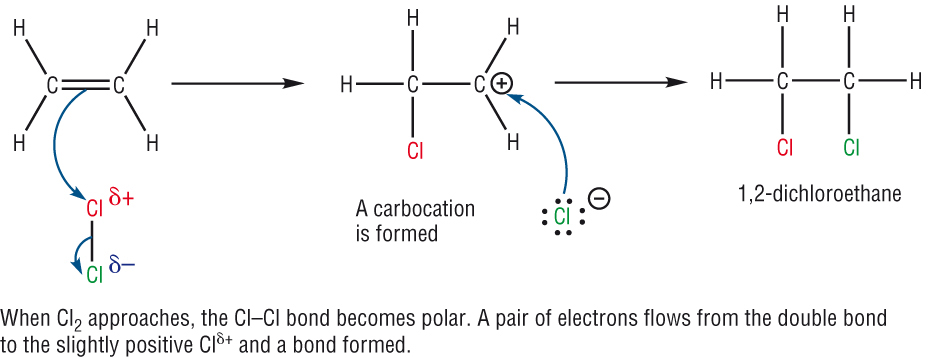
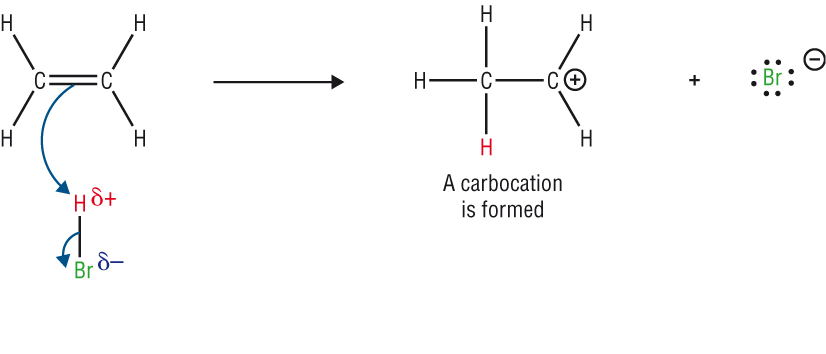
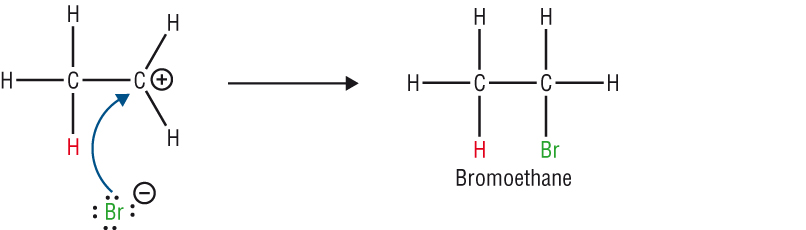
The mechanism:
- The mechanism shows the movement of electrons with arrows.
- In Module 1A: An arrow with a double head shows the movement of a pair of electrons.
 |

.gif)
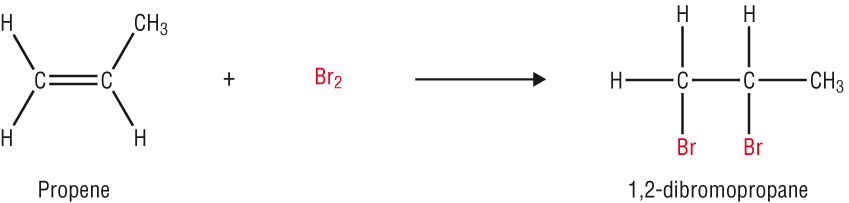
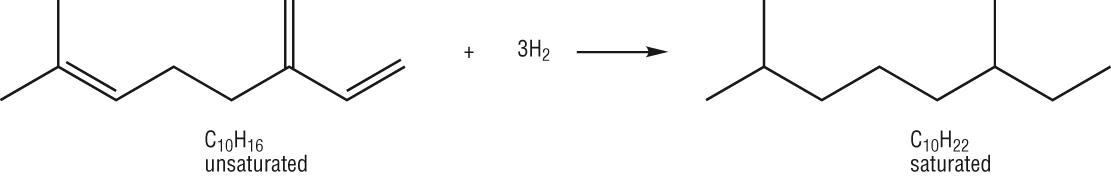
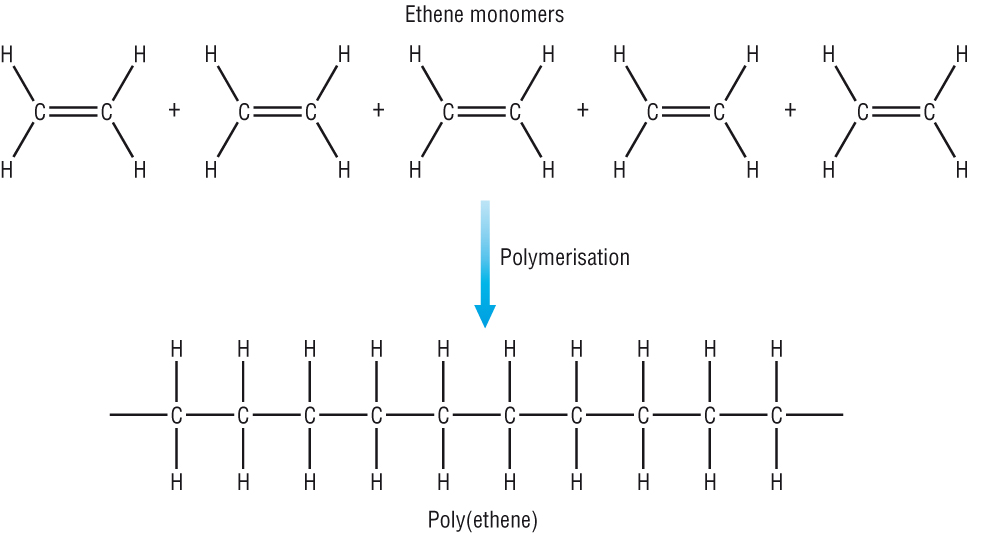
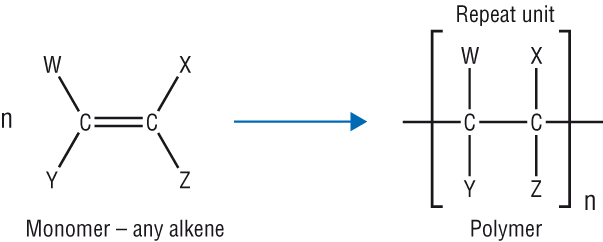
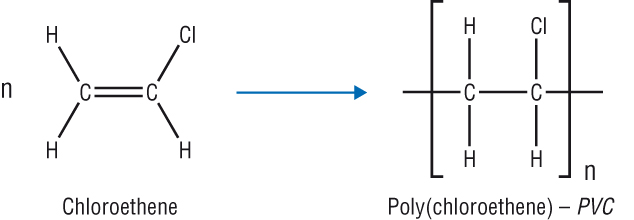
.jpg)
.jpg)
.jpg)