Enthalpy, H
Conservation of energy (First Law of thermodynamics)
Energy cannot be created or destroyed just transferred between the system and the surroundings
| Heat loss in a chemical system | = | Heat gain to the surroundings | Temperature increases | |
| Heat gain in a chemical system | = | Heat loss to the surroundings | Temperature decreases |
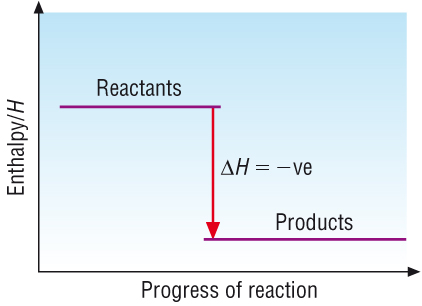
Enthalpy change, DH:
DH = Hproducts - Hreactants
Exothermic reactions:
Hproducts < Hreactants
| DH | = | Hproducts | - | Hreactants |
 |
|
| DH | = | Small | - | Large | ||
| DH | = | Negative value | ||||
|
Energy is released from the system to the surroundings |
||||||
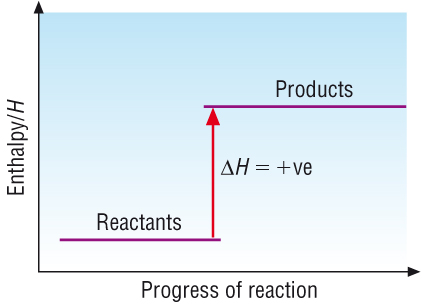
Endothermic reactions:
Hproducts > Hreactants
| DH | = | Hproducts | - | Hreactants |
 |
|
| DH | = | Large | - | Small | ||
| DH | = | Positive value | ||||
|
Energy is transferred to the system from surroundings |
||||||
Questions 1 - 2 P 185
Exothermic and endothermic reactions
1) Oxidation of fuels:
The most common example is the oxidation of methane forming carbon dioxide and water:
| CH4(g) | + | O2(g) | à | CO2(g) | + | H2O(g) | DH | = | - 890 | Kj mol-1 |
The negative sign means that the reaction is exothermic.
This means that Hproducts < Hreactants
The units tell you that 890 Kj of energy is given out per mole of methane.
Changes in enthalpy are given in molar quantities:
Environmental impact - Example:
A small car travelling 2 miles uses 200 - 250g of petrol, C8H18, DH = -5470 KJ mol-1.
How much energy is needed and how much CO2 is produced?
| C8H18(l) | + | 12.5O2(g) | à | 8CO2(g) | + | 9H2O(g) | DH | = | - 5470 | Kj mol-1 |
| 200g | ||||||||||
| Moles = m / Mr | ||||||||||
| Moles = 200 / 114 | ||||||||||
| Moles = 1.75 | 1:8 | |||||||||
| Moles ~ 2 | à | Moles = 16 | ||||||||
| Energy used = 2 x 5470 | Volume = 16 x 24 | |||||||||
| Volume = 384 dm3 |
2) Respiration:
An important exothermic reaction providing energy for all living things:
| C6H12O6(aq) | + | 6O2(g) | à | 6CO2(g) | + | 6H2O(l) | DH | = | -2801 | Kj mol-1 |
Endothermic reactions:
1) Photosynthesis:
An important endothermic reaction without which there would be no life.
This reaction makes 'food', the starting point for all foods, and oxygen for respiration.
Energy from the sun provides the energy to transfer from surroundings to system.
| 6CO2(g) | + | 6H2O(l) | à | C6H12O6(aq) | + | 6O2(g) | DH | = | +2801 | Kj mol-1 |
The reverse reaction of respiration.
2) Thermal decomposition of limestone:
Limestone contains calcium carbonate:
| CaCO3(s) | à | CaCO(s) | + | CO2(g) | DH | = | +178 | Kj mol-1 |
Calcium oxide is commonly known as lime used in cement and used to treat soils by farmers.
If water is added to lime, calcium oxide, heat is given off - exothermic reaction.
Questions 1 - 2 P187
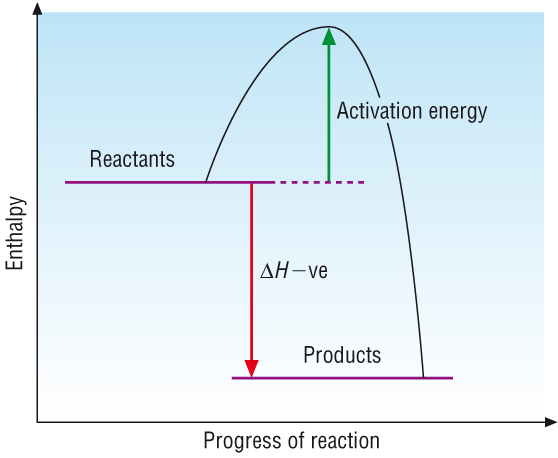
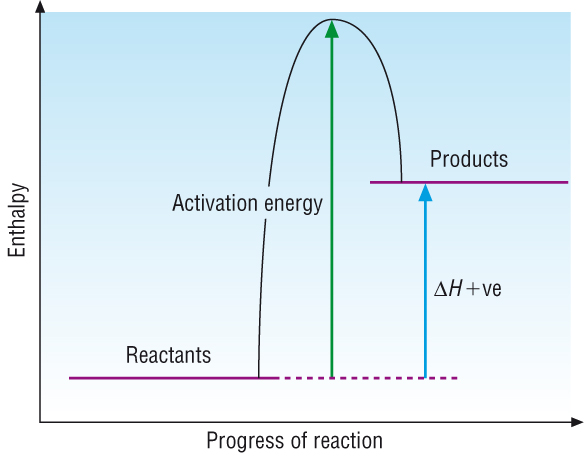
Simple enthalpy profile diagrams:
Reactions and their enthalpy changes have been shown as simple enthalpy profile diagrams:
 |
 |
| Exothermic reactions: Where Hproducts < Hreactants DH = negative |
Endothermic reactions: Where Hreactants > Hproducts DH = positive |
The simple energy profile diagrams assumes that as soon as the reactants come in contact with each other a reaction takes place.
Most reactions do not occur 'spontaneously' but need a little bit of energy to get them going, a spark is needed to set gas alight.
This 'bit of energy' is called the activation energy. It is the energy required to break the bonds in the reactants.
|
Exothermic reactions: |
Endothermic reactions: |
 |
 |
|
|
Questions 1-2 P189
Standards:
Enthalpy changes for reactions will vary slightly depending upon the conditions under which the reaction is carried out.
All data books have to have the same value for enthalpy changes.
Chemists use standard conditions to ensure that all reactions and corresponding enthalpy changes are carried out under the same conditions.
They are as close to normal lab conditions as possible.
Standard conditions:
Pressure at 100 kPa (which is 1 atmosphere)
Temperature at 298K (25oC)
1 Mole or 1 Molar solutions
Normal physical states at standard temperature and pressure (above conditions)
A standard enthalpy change is shown by - DH q
H - Enthalpy
D - Change in
q symbol represents standard conditions
Standard states:
Standard enthalpy changes must have substances in their standard states under these standard conditions:
| Substance | Chemical symbol and state | Explanation |
| Magnesium | Mg(s) | Magnesium is a solid under standard conditions, (s) |
| Hydrogen | H2(g) | Hydrogen is a gas under standard conditions, (g) |
| Water | H2O(l) | Water is a liquid under standard conditions, (l) |
Standard enthalpy changes:
There are 3 standard enthalpy changes that you need to know:
1) Standard enthalpy change of reaction, DHrq
For this enthalpy change we need a reaction to refer to.
A value for the enthalpy change which is true for the molar quantities stated in the reaction:
| H2(g) | + | 1/2 O2(g) | à | H2O(l) | DHrq | = | -286 KJMol-1 |
For the reaction:
Fe2O3(s) + 2Al(s) à 2Fe(s) + Al2O3(s) DHqr = -851.50 KJ Mol-1
For the same reaction with different stoichiometry:
1/2Fe2O3(s) + Al(s) à Fe(s) + 1/2Al2O3(s) DHqr = -425.75 KJ Mol-1
Note that the reaction must be written in order for the information to be correct. DHqr is half the value if the reaction is balanced using halves.
2) Standard enthalpy of combustion – DHqc
Combustion reactions are so common that they have a standard enthalpy change of their own:
Definition: The enthalpy change that occurs when 1 mole of a substance reacts completely with oxygen under standard conditions. All reactants and products are in their standard states.
DHqc for ethane:
| C2H6(g) | + | 3.5 O2(g) | à | 3H2O(l) | + | 2CO2(g) | DHcq | = | -1560 KJMol-1 |
DHqc refers to the complete combustion of 1 mole of ethane.
This conforms with the definition -' when 1 mole of a substance reacts completely with oxygen'
3) Standard enthalpy change of formation, DHqf
Formation reactions are also so common that they have a standard enthalpy change of their own:
Is the enthalpy change that takes place when 1 mole of a compound is formed from its constituent elements in their standard states under the standard conditions.
DHqf for water:
H2(g) + 1/2O2(g) à H2O(l) DHqf = -286 KJ Mol-1
This refers to the Standard enthalpy change of formation of 1 mole of water.
This conforms with the definition -' when 1 mole of a compound is formed from its elements'
A problem:
If we are forming an element H2, there is no chemical change.
We say that elements in their standard states under standard conditions have a standard enthalpy of 0 KJ Mol-1
Questions 1 - 3 P191
Determination of enthalpy changes
Enthalpy content of reactants and products cannot be measured directly.
You can measure the enthalpy change between the reactants and products - enthalpy change for a reaction, DHr
Remember:
| Heat loss in a chemical system | = | Heat gain to the surroundings | Temperature increases | |
| Heat gain in a chemical system | = | Heat loss to the surroundings | Temperature decreases |
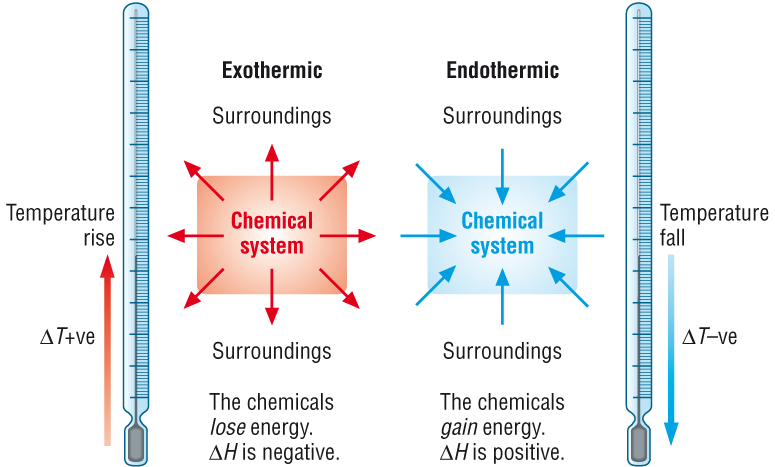
The enthalpy change for any reaction, DHr is measured in KJ Mol-1, which is energy per mole
This means we need to know 2 things
i) The energy change - for which we use temperature change
ii) The amounts in moles of the limiting reagent that reacts
i) The energy change:
To determine the energy change we use the formula:
Q = mcDT
1000
Q – quantity of energy exchanged J, the 1000 converts to kJ
m – mass of the water g (ie cm3 as the density if water is 1 gcm-3)
c – specific heat capacity j g-1 K-1
DT – rise in temperature K, (Tinitial - Tfinal)
Convert the energy calculated into KJ (divide by 1000).
ii) Calculate the number of moles used:
No Moles = Mass or c x V
Mr
iii) Calculate the amount of energy exchanged per mole , this is the enthalpy:
Enthalpy = Energy
Moles
Check you have the sign correct:
(-)ve for exothermic reactions
(+)ve for endothermic reactions
Direct determination of enthalpy changes:
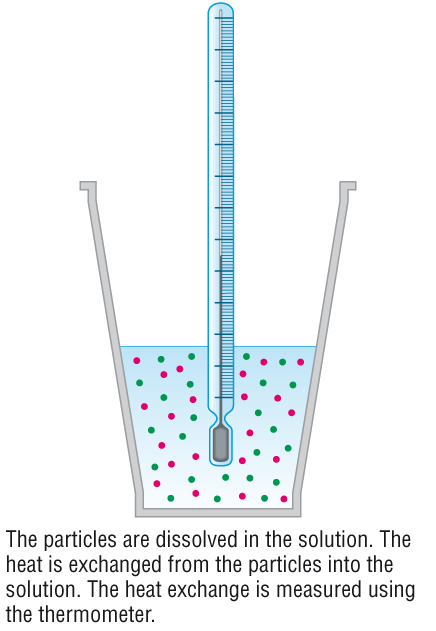 |
|
Example:
Excess Mg is added to 100cm3 of 2.00 Mol dm-3 CuSO4, the temperature rose from 20.0oC to 65oC.
i) The energy change:
| Q | = | (m | x | c | x | DT) / 1000 | |
| Q | = | (100 | x | 4.18 | x | 45) / 1000 | |
| Q | = | 18.81 kJ | Temp increases, it is exothermic therefore is negative | ||||
| Q | = | - 18.81 kJ | |||||
ii) Calculate the number of moles used:
No Moles = c x V
No Moles = 2 x 0.100
No Moles = 0.2
iii) Calculate the amount of energy exchanged per mole , this is the enthalpy:
Enthalpy = Energy
Moles
Enthalpy = - 18.81
0.2
Enthalpy = - 94.05 Kj Mol-1
Finally write the equation with the enthalpy change:
| Mg(s) | + | CuSO4(aq) | à | MgSO4(aq) | + | Cu(s) | DHrq | = | - 94.05 KjMol-1 |
Questions 1 - 2 P193
This is any reaction involving oxygen forming oxides:
| CH4(g) | + | 2O2(g) | à | CO2(g) | + | H2O(l) | DHcq | = | - 890 KjMol-1 |
| H2(g) | + | 1/2O2(g) | à | H2O(g) | DHcq | = | - 286 KjMol-1 | ||
| Al(s) | + | 3/4O2(g) | à | 1/2 Al2O3(s) | DHcq | = | - 1676 KjMol-1 |
Experimental determination of DHc
The enthalpy change of combustion, DHc is also measured in KJ Mol-1, which is energy per mole
This means we need to know 2 things
i) The energy change - for which we use temperature change when heating water
ii) The amounts in moles of fuel used
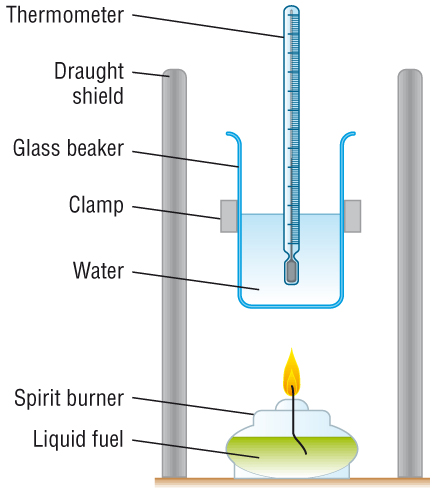 |
|
Example:
1.5g of propan-1-ol heated 250cm3 of water by 45oC.
i) The energy change:
| Q | = | (m | x | c | x | DT) / 1000 | |
| Q | = | (250 | x | 4.18 | x | 45) / 1000 | |
| Q | = | 47.025 kJ | Temp rises, it is exothermic therefore is negative | ||||
| Q | = | - 47.025 kJ | |||||
ii) Calculate the number of moles used:
No Moles = Mass
Mr
No Moles = 1.5
60
No Moles = 0.025
iii) Calculate the amount of energy exchanged per mole , this is the enthalpy:
Enthalpy = Energy
Moles
Enthalpy = - 47.025
0.025
Enthalpy = - 1881 Kj Mol-1
Comparison of experimental value with standard enthalpy change:
| Standard enthalpy change of combustion of C3H7OH, DHcq | - 2021 Kj Mol-1 |
| Experimental enthalpy change of combustion of C3H7OH, DHc | - 1881 Kj Mol-1 |
Errors:
Incomplete combustion of the fuel - less heat energy given out
Heat loss to the surroundings - less heat energy measured
Improvements:
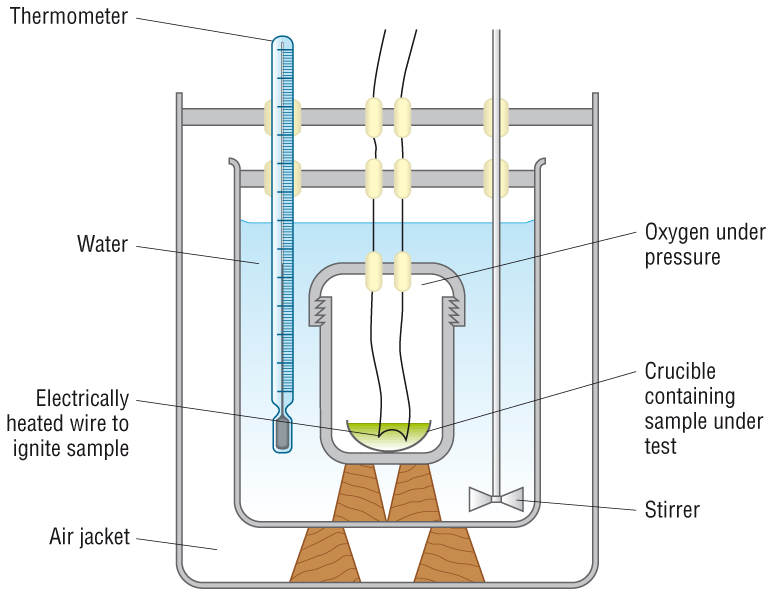 |
|
Questions 1-2 P195
Bond enthalpy:
Is the enthalpy change that takes place when breaking by homolytic fission1 mole of a given bond in the molecules of a gaseous species
To summarise - it is the energy required to break 1 mole of bonds in the gaseous state.
| Breaking bonds | = | Energy is put in to break bonds | Endothermic process | |
| Forming bonds | = | Energy is released when bonds are formed | Exothermic process |
Some examples:
| H - H(g) | à | 2H(g) | DH | = | +436 KjMol-1 | ||
| H - Cl(g) | à | H(g) |
+ |
Cl(g) | DH | = | + 432 KjMol-1 |
These bonds enthalpies can only exist in these examples but some bonds can exist in very different molecules.
In cases like this we use the Average bond enthaply
Average bond enthalpy:
Bonds like C - H exist in all hydrocarbons but their bond enthalpies will vary depending on their environment:
|
|
|
The C - H bond enthalpy will be different in an alkane than the C - H bond enthalpy in an aldehyde where the C atom is adjacent to an oxygen atom.
For bond enthalpies that are common, we use average bond enthalpies:
| Bond | Average bond enthalpy / KJ Mol-1 |
| C - H | +413 |
| O = O | +497 |
| O - H | +463 |
| C = C | +612 |
| H - H | +436 |
 |
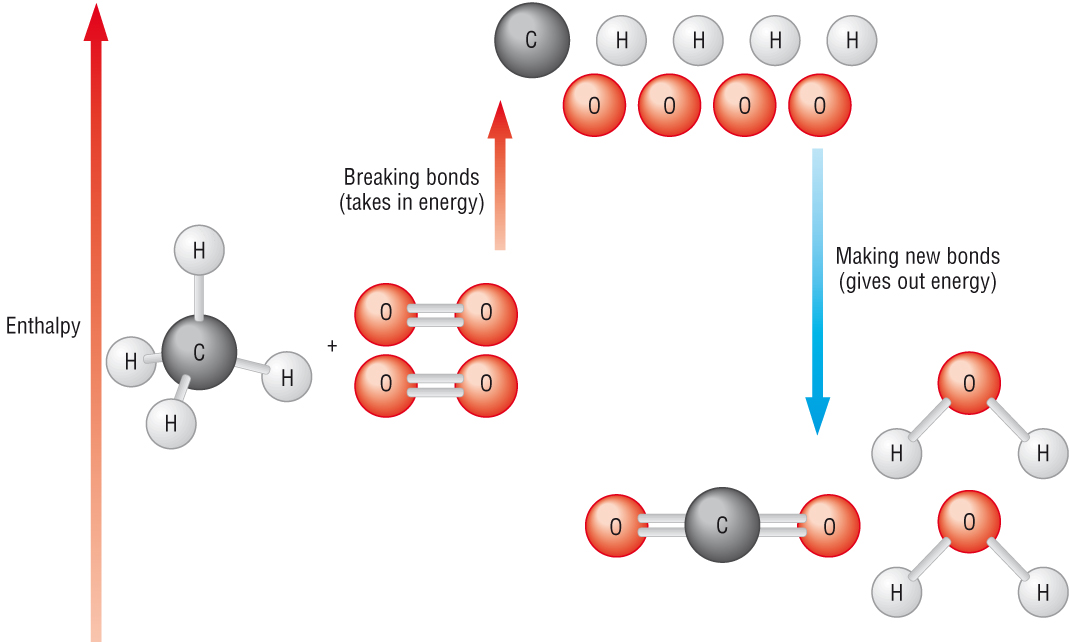 |
In this and any reaction, reactant bonds will be broken and new product bonds will be formed.
When bonds are broken energy is required making it an endothermic process, the activation energy.
When new bonds are formed energy is release making it an exothermic process.
If the energy released when new bonds form making the products is greater than the energy needed to break the bonds of the reactants, the reaction is exothermic.
If the energy released when new bonds form making the products is less than the energy needed to break the bonds of the reactants, the reaction is endothermic.
Strong bonds require lots of energy to break but they also release lots of energy when they form:
| Breaking strong bonds | à | Forming weak bonds | Endothermic process | |
| Breaking weak bonds | à | Forming strong bonds | Exothermic process |
Using bond enthalpies to determine enthalpy changes:
The symbol S is used for 'sum of' or adding together
| Energy required to break bonds | = | S (Bond enthalpies of bonds broken) | ||
| Breaking weak bonds | = | S (Bond enthalpies of bonds formed) |
Formula:
| DH | = | S (Bond enthalpies of bonds broken) |
- |
S (Bond enthalpies of bonds formed) |
Worked example:
|
|
à |
|
à |
|
| S (Bond enthalpies of bonds broken) | S (Bond enthalpies of bonds formed) | |||
|
Reactants |
Products |
||||||
|
Bond |
Number |
Bond energy |
Total |
Bond |
Number |
Bond energy |
Total |
|
C - H |
4 |
413 |
1652 |
C = O |
2 |
805 |
1610 |
|
O = O |
2 |
497 |
994 |
O - H |
4 |
463 |
1852 |
| S (Bond enthalpies of bonds broken) = |
2646 |
S (Bond enthalpies of bonds formed) = |
3462 |
||||
| DH | = | S (Bond enthalpies of bonds broken) |
- |
S (Bond enthalpies of bonds formed) |
| DH | = | 2646 |
- |
3462 |
| DH | = | - 816 Kj Mol-1 |
Questions 1-2 P197
Enthalpy changes from DHcq - Hess's law
Measuring enthalpy changes indirectly:
Sometimes it is not possible to measure an enthalpy change directly.
This may be due to:
High activation energy
Slow rate of reaction
More than one reaction occurring at the same time
Hess's law allows us to work out enthalpy changes that are not possible to measure:
The total enthalpy change accompanying a chemical change is independent of the route by which the chemical change takes place provided the initial and final conditions are the same.
.jpg)
If this was not true, it could be possible to gain energy via route A instead of going via route B. This would break the 1st law of thermodynamics.
It is found that reactants can be converted into the same products by more than 1 route.
The total energy for each route is the same: These are called Enthalpy cycles.
Using Hess’s law: Enthalpy cycles
Enthalpy cycles are used to determine enthalpy changes for reactions that are not easily measured directly.
This can be done in 2 ways:
1. Enthalpy changes of combustion
2. Enthalpy changes of formation
1 Calculating
DHq from enthalpy changes of combustion:
![]()
Consider the enthalpy change below:
3C(s) + 4H2(g) à C3H8(g)
Combustion reactions can be used to find this enthalpy change as carbon hydrogen and propane all burn in oxygen.
This means that their enthalpy changes of combustion can all be measured
Constructing an enthalpy cycle:
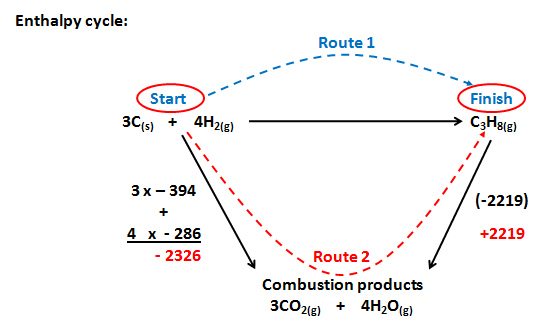
we use the opposite sign for - 2219 as our route, Route 2 goes the opposite way to the enthalpy change
Route 2 = - 2326 + + 2219
Route 2 = - 107 Kj Mol-1
Remember
Route 1 = Route 2
Route 1 = - 107 Kj Mol-1
Using the formula:
| DH | = | S DHcq (reactants) |
- |
S DHcq (products) |
| DH | = | (3 x - 394) + (4 x - 286) |
- |
- 2219 |
| DH | = |
- 107 Kj Mol-1 |
Question 1 P199
Enthalpy changes from DHqf - Hess's law
2) Using enthalpy change of formation
![]()
As with enthalpy changes of combustion, enthalpy changes of formation can be used in Hess's cycles to work out enthalpy changes.
Consider the reaction:
2SO2(g) + O2(g) à 2SO3(g)
The enthalpy cycle would be:
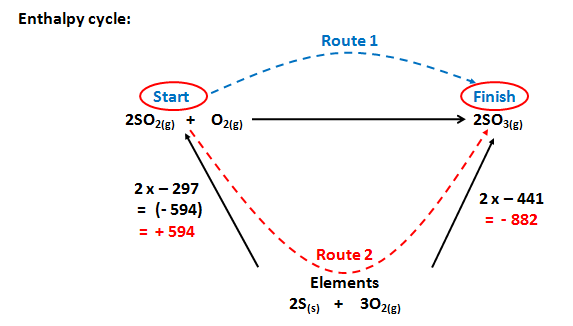
we use the opposite sign for - 594 = + 594 as our route, Route 2 goes the opposite way to the enthalpy change
Route 2 = + 594 + - 882
Route 2 = - 288 Kj Mol-1
Remember
Route 1 = Route 2
Route 1 = - 288 Kj Mol-1
Using the formula:
| DH | = | S DHfq (products) |
- |
S DHfq (reactants) |
| DH | = | (2 x - 441) |
- |
(2 x - 297) |
| DH | = |
- 288 Kj Mol-1 |
Other enthalpy cycles:
As long as there is a link between reactants and products, Hess's law can be applied and enthalpy cycles can be constructed.
Follow the principles as outlined above and you won't go wrong.
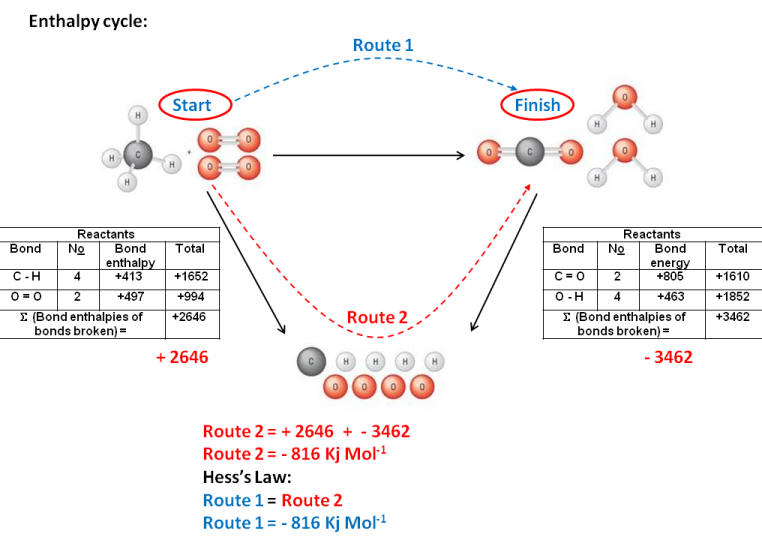
Alternative approach
using bond enthalpies:
![]()
Review: Using the Formula:
| DH | = | S (Bond enthalpies of bonds broken) |
- |
S (Bond enthalpies of bonds formed) |
Worked example:
|
|
à |
|
à |
|
| S (Bond enthalpies of bonds broken) | S (Bond enthalpies of bonds formed) | |||
|
Reactants |
Products |
||||||
|
Bond |
Number |
Bond energy |
Total |
Bond |
Number |
Bond energy |
Total |
|
C - H |
4 |
413 |
1652 |
C = O |
2 |
805 |
1610 |
|
O = O |
2 |
497 |
994 |
O - H |
4 |
463 |
1852 |
| S (Bond enthalpies of bonds broken) = |
2646 |
S (Bond enthalpies of bonds formed) = |
3462 |
||||
| DH | = | S (Bond enthalpies of bonds broken) |
- |
S (Bond enthalpies of bonds formed) |
| DH | = | 2646 |
- |
3462 |
| DH | = | - 816 Kj Mol-1 |
Questions 1-2 P197
Summary of enthalpy cycles
Step 1:- Write a balanced chemical equation for the reaction.
Step 2:- Construct the Enthalpy cycle.
Step 3:- Decide on your routes and draw them on the cycle
Step 4:- Write in the DHq for each compound / element next to the arrows.
Step 5:- Look up the values of each DHq and write them in. Add them up for each route.
Step 6:- Write out Hess’s law – Route 1 = Route 2
Step 7:- Put in your numbers.
Step 8:- Calculate DHq
Question 1-2 P201 / 1-7 P215 / 1,3-5 P217