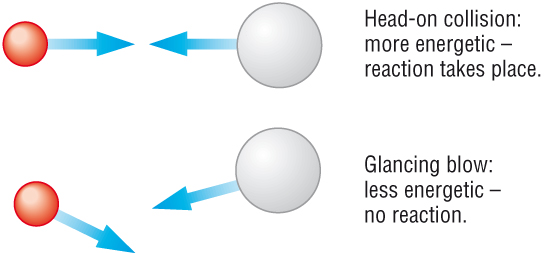
Rates of reaction - collision theory
Introduction:
The term rate is used to describe the speed of a reaction.
Different reaction will have different rates
Rate of reaction:
The rate of a reaction is defined as the change in concentration of a reactant or product in a given time
| Rate | = | Change in concentration | Units: | mole dm-3 | = | mol dm-3 s-1 | |||
| Time | s |
When a reaction starts the concentrations of the reactants are at their highest, the rate is at its fastest.
As the reactants are used up the concentration decreases, so does the rate.
When one of the reactants is used up the reaction stops. The rate is 0/
Factors affecting the rate of a chemical reaction:
| Increase in concentration | |
| Increase in No particle per volume | |
| Increase in the collision frequency | |
| Increases the rate of the reaction |
| Increase in pressure | |
| Decrease in the volume | |
| Increase in the No particle per volume | |
| Increase in the collision frequency | |
| Increases the rate of the reaction |
| N2(g) | + | 3H2(g) | D | 2NH3(g) |
| Protease | Breaks down protein stains | |
| Amylase | Breaks down starches | |
| Lipases | Breaks down fats and greases |
| CH3CH2OH | + | 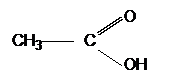 |
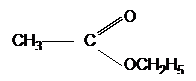 |
+ | H2O |
The hydrogen ions from the acid is in the same phase as the reactants.
The hydrogen ion becomes involved in the reaction mechanism but the concentration of the acid will be the same at the end.
| Some will have high energy: | This means they will move very fast | |
| Some will have low energy | This means they will move very slow | |
| Most will have average energy | Most will move with the average speed |
| The area under the curve is equal to the total number of molecules in the sample: | The area does not change | |
| No molecules have zero energy: | The curve starts at 0,0 | |
| There is no maximum energy for a molecule: | The curve never touches the axis | |
| Only molecules with energy greater than the activation energy are able to react | The activation energy is the minimum energy required to react |
| Increase in temperature | Increases kinetic energy of molecules | |
| Increase in kinetic energy of molecules | Increases the speed of the molecules | |
| Increase in the speed of the molecules | - Increases the collision frequency | |
| - Increases the No of collisions with energy > activation energy | ||
| - Increases the collision frequency | More successful collisions | |
| - Increases the No of collisions with energy > activation energy | ||
| More successful collisions | Increase in the rate |
| Catalysts offer a route with a lower activation energy | More molecules will overcome the lower activation energy | |
| As more molecules will overcome the lower activation energy | Increases the number of successful collisions | |
| An increases in the number of successful collisions | Increases the rate of the reaction |
Questions 1-3 P209
Reversible reactions:
| Mg(s) | + | H2SO4(aq) | à | MgSO4(aq) | + | H2(g) |
| 2SO2(g) | + | O2(g) | D | 2SO3(g) |
| 2SO3(g) | D | 2SO2(g) | + | O2(g) |
Dynamic equilibrium:
Fixed conditions: Temperature and pressure remains the same.
Isolated system: No material is added or removed from the system
Factors affecting the position of equilibrium:
1) Changing the concentration of reactants or products
2) Changing the pressure (if gases involved)
3) Changing temperature
1) Changing the concentration of reactants or products
This reaction shows how a reversible reaction can be manipulated by changing the concentration of the reactants then products:
Co(H2O)62+ + 4Cl-(aq) D CoCl42-(aq) + 6H2O(aq)
Adding HCl to the reaction mixture increases the concentration of Cl- ions.
The equilibrium shifts towards the products in order to reduce the concentration of the Cl- ions.
The resulting colour change is from pink to blue.
At this point if you add H2O molecules to the reaction mixture, the Cl- concentration decreases
The reaction moves towards the reactants in order to increase the Cl- ion concentration..
The resulting colour change is from blue to pink.
Concentration Summary:
An increase in the concentration of the reactants moves the equilibrium towards the products. The equilibrium removes the extra reactants by shifting the equilibrium in the forward direction.
An increase in the concentration of the products moves the equilibrium towards the reactants. The equilibrium removes the extra products by shifting the equilibrium in the reverse direction.
2) Changing the pressure (if gases involved)
The syringe contains 2 gases in equilibria:
N2O4 D 2NO2
3) Changing temperature:
a) Ammonium chloride
The white solid vaporises on heating into NH3(g) and HCl(g).
Further up the tube when the vapour has cooled NH4Cl(s) reforms.
This is an example of a reversible reaction:-
NH4Cl(s) à NH3(g) + HCl(g) On heating
NH3(g) + HCl(g) à NH4Cl(s) On cooling
A change in temperature affects the position of the equilibrium
NH4Cl(s) D NH3(g) + HCl(g) DH = endothermic
Heating the reaction moves it to the products. The cold side – endothermic.
Cooling moves the reaction to the reactants. The hot side – exothermic.
b) Nitrogen oxides:
The syringe contains 2 gases in equilibria:
N2O4 D 2NO2 DHq = +58KJ Mol-1
N2(g) + 3H2(g) D 2NH3(g) DH = -92KjMol-1