Introduction to aromatic chemistry
This topic considers the chemistry of a group of hydrocarbons called Arenes.
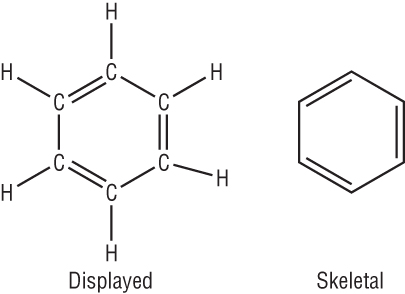
Benzene is usually shown as a hexagon with a circle inside:

Benzene is the simplest arene, C6H6.
These compounds are often described as aromatic due to their 'aroma' or fragrant smell.
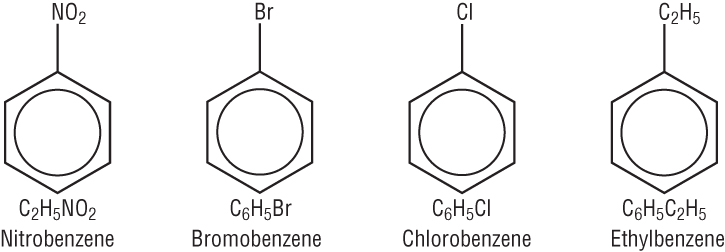
Naming compounds based on benzene:
For a group to be attached to a benzene ring, a hydrogen must be replaced.
Common groups are - Cl chloro, - Br Bromo, - NO2 Nitro, and alkyl groups, - CH3 Methyl:
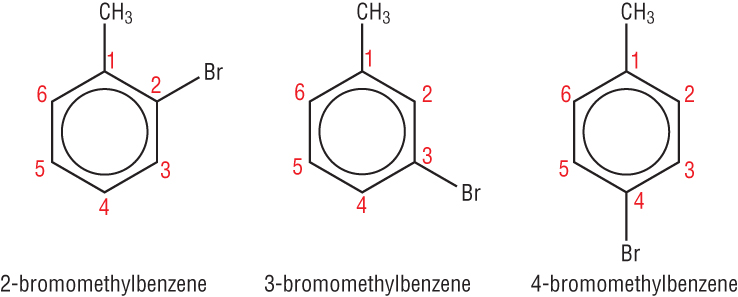
If there is more than 1 group attached then we number to give the lowest value around the ring.
We start numbering at the alkyl group / major functional group:
Benzene:
|
 |
The structure of benzene:
Faraday determined that the empirical formula was CH.
It was later found that the Mr = 78. This suggested that the molecular formula was C6H6.
The structure of benzene provided chemists with a major problem.
The first structure suggested was:
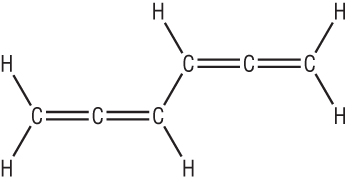
The C=C suggests that benzene should be very reactive but in fact is very unreactive.
Kekule suggested a ring structure after dreaming of snakes eating their tales:
Questions 1-5 P5
Problems with Kekule structure:
Although Kekule's structure explained the ring structure and the formula C6H6, there were still some chemical and physical properties that could not be explained by this structure.
Benzene's low reactivity:
Kekule's structure failed to explain the low reactivity of benzene.
According to the Kekule structure, 3 double bonds would be as (if not more) reactive as the alkenes.
It is in fact quite unreactive compared to alkenes.
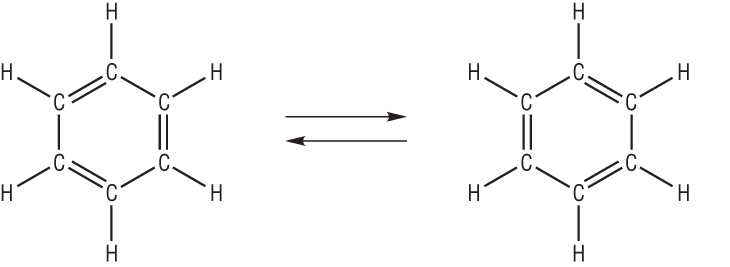
Kekule's equilibrium model of benzene
Kekule suggested that this was due to an equilibria which involved the double bonds switching positions.
He explained that the C=C changed position before any reactions could occur:
The carbon - carbon bond length in benzene:
X - Ray diffraction threw some light on the structure.
It was found that all the C-C bond lengths were equal in length.
Kekule's structure would not be:
 |
Or |  |
C – C 0.153nm cyclohexane
C = C 0.134nm cyclohexene
C – C 0.139nm benzene
This suggests that the C - C bond is somewhere between a single and double bond
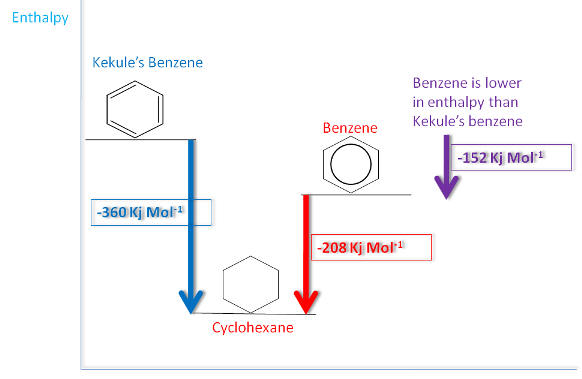
Hydrogenation of benzene
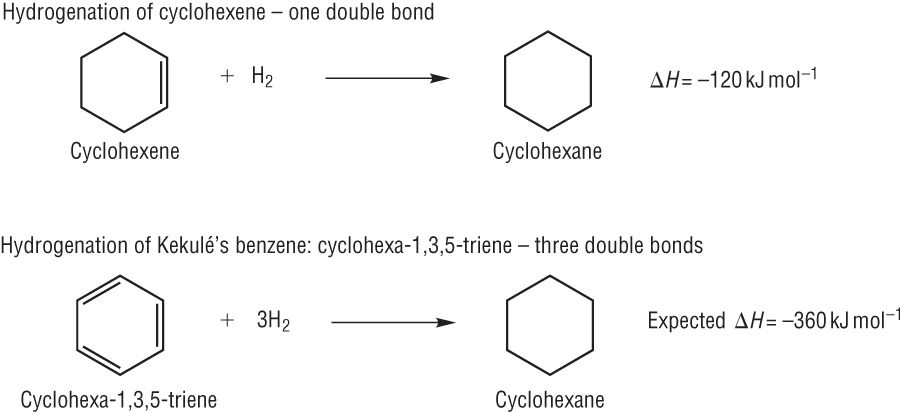
Compare the results with the known value for Benzene:-
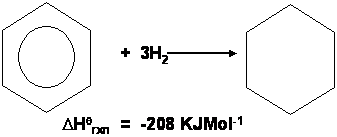
This tells us that benzene is more stable than the Kekule model as less energy is given out:
Qu 1 - 3 P 7
The delocalised model of benzene
The delocalised model of benzene
The weaknesses of the Kekule model lead to the delocalised model for benzene.
Benzene has the following properties which need explaining:
| 1) | 6 carbon's and 6 hydrogen's. | |
| 2) | Arranged in a hexagonal ring. | |
| 3) | The shape around each carbon atom is trigonal planar with a bond angle of 120o. | |
| 4) | Carbon carbon bond lengths are all the same. |
This can only be explained if we look at the bonding in benzene around the carbon atoms:
|
|
P orbital |
|
| s bond with p orbitals overlapping to form a p bond in alkenes |
Imagine 6 of these p orbitals on every carbon in benzene:
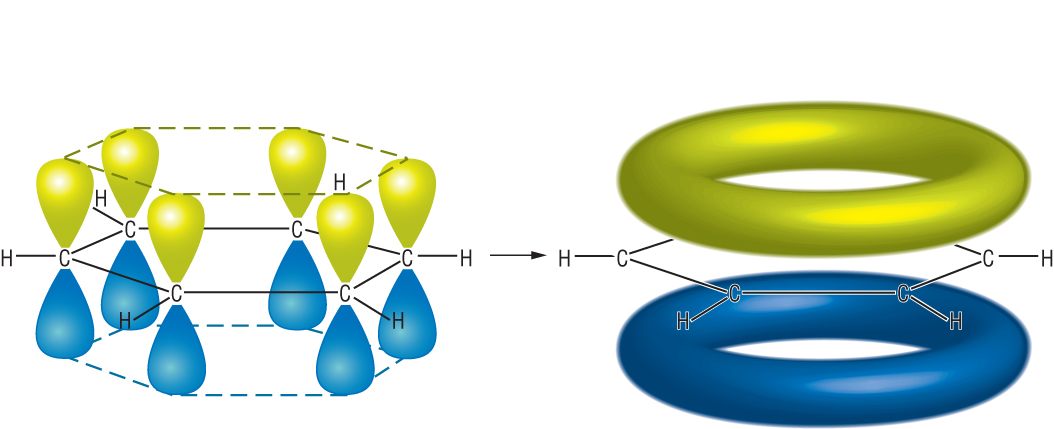 |
|
|
| 6 x p orbitals | p delocalised orbital | |
6 electrons are delocalised in this system.
This means that each C - C has 3 electrons. 2 in the s bond and 1 from the delocalised p system.
This makes it slightly less electron rich than alkenes which means that they are not as good at polarising electrophiles.
The bonding in benzene cannot therefore be described as 3 C = C and 3 C – C bonds.
One way of thinking about it is that the bonds between the carbons is somewhere in between (6 x 1 1/2 bonds!!)
The bonding but must be considered as a delocalised electron charge cloud spread out over the whole ring.
This gives rise to certain difficulties when drawing the structure of benzene as:-
= Represents 4e’s in a double bond
As neither of these is appropriate to show the distribution of electrons in benzene, the following structure is often used:-
 |
The delocalised model of benzene and chemical reactivity:
Benzene is more stable than expected, less so than alkenes.
This is because the electron density between the carbon atoms is less than in alkenes.
This makes them attract electrophiles less well than alkenes:
| Reaction with | Alkenes | Benzene |
| Decolorise bromine water | ü | û |
| Strong acids, HCl | ü | û |
| Halogens, Cl2 | ü | û |
If it was an addition reaction like the alkenes then the delocalised ring structure would be disrupted as the electrons would be needed to form bonds with the electrophile.
This would make the product less stable than benzene meaning the reaction would be energetically unfavourable.
For these reasons, benzene undergoes substitution instead of addition in order to maintain the delocalised electron system and its stability.
Qu 1 - 4 P 9
Reactivity
Benzene in toxic and carcinogenic so Methylbenzoate (or methoxy benzene), a derivative of benzene is used:-
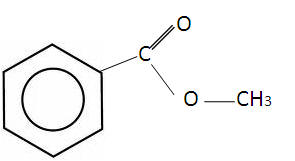
Electrophilic substitution
Methoxybenzene is slightly more reactive than benzene. The conditions involving benzene will be more severe.
1) Nitration of benzene
Nitration of benzene requires the production of a more reactive nitryl ion:
HNO3 + H2SO4 à NO2+ + HSO4- + H2O
|
|
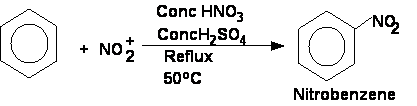 |
+ H+ |
The H+ generated will react with HSO4- forming sulphuric acid, H2SO4. This means sulphuric acid is a catalyst
| H+ | + | HSO4- | à | H2SO4 |
The reaction fro the nitration of benzene is:
| H2SO4 | ||||||
| C6H6 | + | HNO3 | à | C6H5NO2 | + | H2O |
| 50oC |
Mechanism:
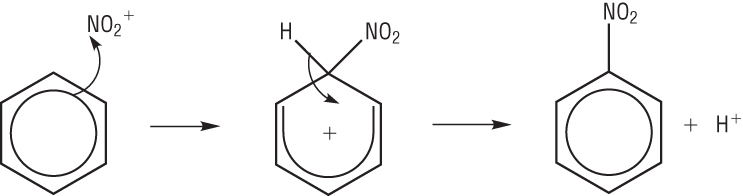
The benzene ring is rich with electrons which mean the ring will attract an electrophile.
A pair of electrons move from the ring to the nitryl ion forming a covalent bond.
This disrupts the delocalisation of the benzene ring to 4 electrons over the remaining 5 carbon atoms leaving a positive charge.
The loss of the hydrogen (proton) puts 2 electrons back into the benzene. This restores the full delocalised p electron system.
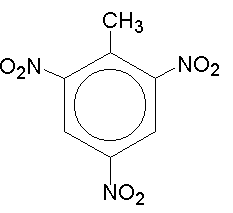
Nitration of methylbenzene
Methyl benzene used to be called toluene.
The nitration of toluene forms nitro toluene.
This can be nitrated in the 2,4,6 position:
2) Halogenation of benzene
Benzene will not react with bromine on its own. It requires the help of a type of catalyst called a halogen carrier.
These halogen carriers include:
FeCl3, FeBr3, AlCl3, AlBr3 (depending on which halogen you are adding)
Fe can be used on its own as it will react with any halogen forming FeHal3
These reactions involve electrophilic substitution (as with nitration) where a hydrogen is substituted with a halogen.
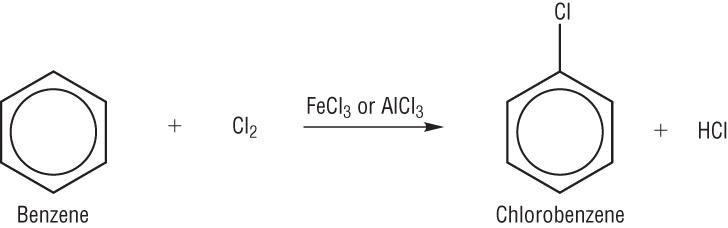
When a benzene ring reacts with chlorine, chlorobenzene and hydrogen chloride is produced.
When a benzene ring reacts with bromine, bromobenzene and hydrogen bromide is produced.
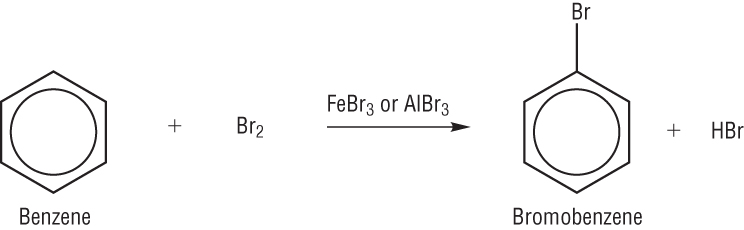
Bromobenzene is used in production of pharmaceuticals.
Similarly the reaction with chlorine (and FeCl3) gives chlorobenzene and hydrogen chloride.
Chlorobenzene is used in the production of pesticides.
Function of the halogen carrier (catalyst):
Benzene is more stable than alkenes which means that Br2 is not a strong enough electrophile.
Although benzene has delocalised electrons, it is not sufficiently electron rich to polarise a bromine molecule like an alkene (count the electrons!)
The halogen carrier generates a Br+ which is a more powerful electrophile than a bromine molecule (because it is more positive)
Mechanism:
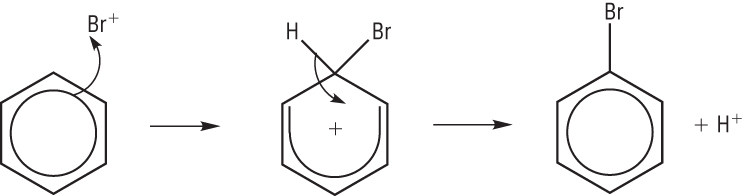
The benzene ring is rich with electrons which mean the ring will attract an electrophile.
A pair of electrons move from the ring to the bromonium ion forming a covalent bond.
This disrupts the delocalisation of the benzene ring to 4 electrons over the remaining 5 carbon atoms leaving a positive charge.
The loss of the hydrogen (proton) puts 2 electrons back into the benzene. This restores the full delocalised p electron system.
Regeneration of the halogen carrier (catalyst):
The hydrogen ion generated reacts with FeBr4- regenerating the halogen carrier catalyst and hydrogen bromide
Other halogens
The reactions and mechanisms are the same for all halogenation reactions, Cl2 with FeCl3 etc.
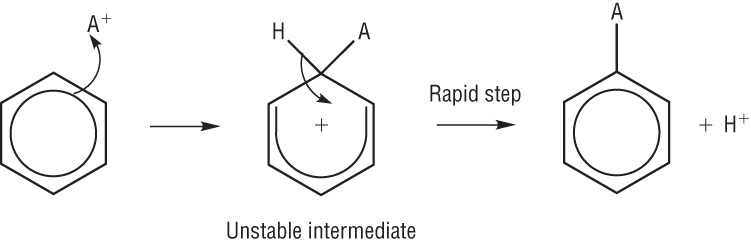
Mechanisms for benzene:
All the reactions involving benzene undergo the same mechanism:
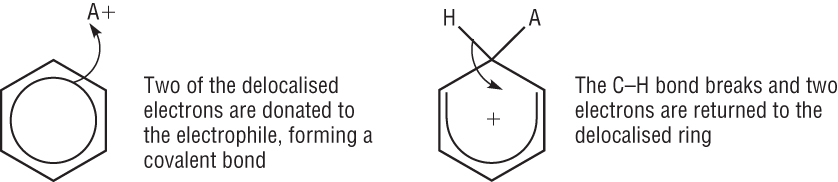
The benzene ring is electron rich which means the ring will attract an electrophile.
A pair of electrons move from the ring to the electrophile forming a covalent bond.
This disrupts the delocalisation of the benzene ring to 4 electrons over the remaining 5 carbon atoms leaving a positive charge.
The intermediate is unstable meaning the second step is rapid
The loss of the hydrogen (proton) puts 2 electrons back into the benzene. This restores the full delocalised p electron system.
Full mechanism:
Qu 1 - 4 P11 / 1 - 3 P13
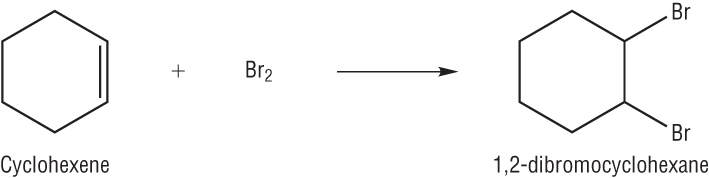
The reactivity of alkenes and benzene
Cyclohexene vs Benzene
| Cyclohexene | Benzene |
|
|
 |
 |
| The difference between an alkene and benzene is the electron density | |
|
|
|
|
|
| The
mechanism:
|
The mechanism: |
|
|
| Electrophilic addition | Electrophilic substitution |
Qu 1-2 P15
In alcohols the OH group is attached to an alkyl group.
In phenol, the OH group is attached directly to the benzene ring.
Phenols are used in antiseptics.
It is described as an aromatic alcohol:
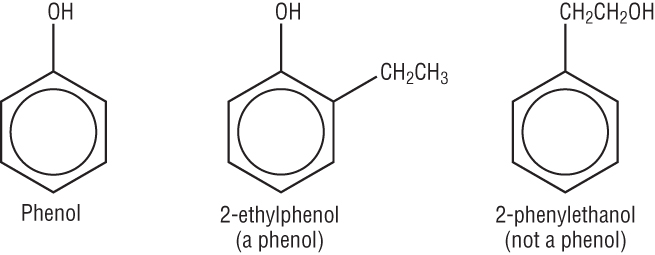
Phenol is toxic and corrosive therefore a safer alternative is used which will show the chemistry of phenol. Methyl 4-hydroxybenzoate is used as a safe alternative:
Phenol is sparingly soluble in water.
The OH in phenol will form hydrogen bonds just like ethanol.
The large benzene ring limits its solubility only being able to form weak van der Waals.
Phenol is a weak acid because it partially dissociates in water:

Phenol Phenoxide
Acid + metal hydroxide à salt + water
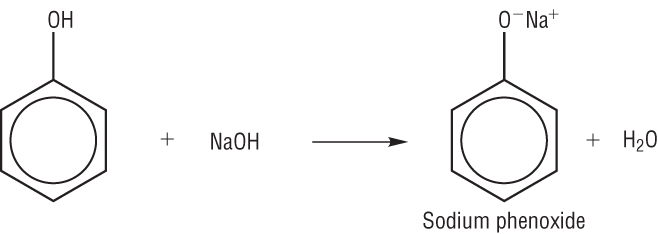
C6H5OH + NaOH à C6H5O-Na+ + H2O
b) Reaction with sodium:
Acid + metal à salt + hydrogen
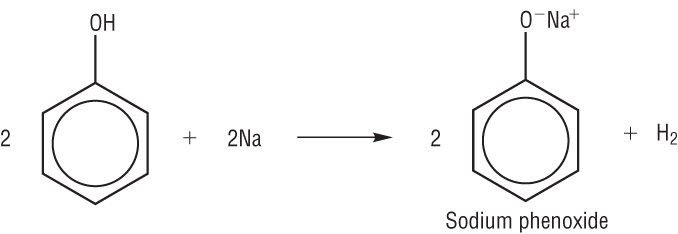
2C6H5OH + 2Na à 2C6H5O-Na+ + H2
This gives the benzene ring in phenol a high electron density = electron rich, the ring is said to be activated.
This will polarise and attract an incoming electrophile like bromine sufficiently that it will react readily.
So phenols do not require a halogen carrier.
Uses of phenols:
Antiseptics
Synthesis of dyes and aspirin
Explosives
Resins (plastic like materials, and / or glues).
Qu 1 - 3 P17 / 1 - 3 P19
Qu 1- 4 P41
Qu 1 - 3 P43