.gif)
.gif)
.gif)
.gif)
Something fishy about amines:
Have an NH2, amine group.
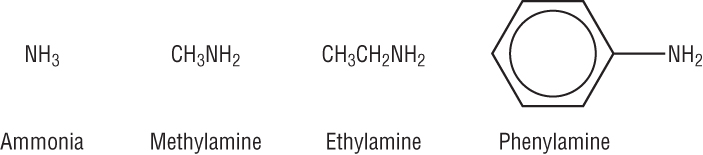
Amines are derivatives of ammonia:
.gif) |
.gif) |
.gif) |
.gif) |
| 3 H atoms | 1 H atom replaced: 1 attached C to N | 2 H atoms replaced: 2 attached C's to N | 3 H atom replaced: 3 attached C's to N |
| Ammonia, NH3 | Primary amine | Secondary amine | Tertiary amine |
Amines occur in nature and are known for their physiological effects:
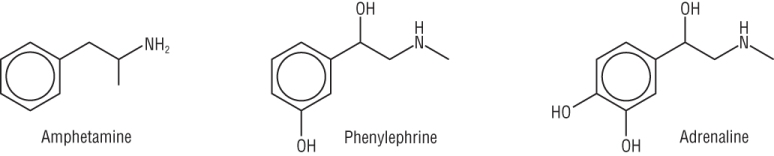 |
||
| Treats drowsiness and fatigue syndrome | Decongestant | 'Fight or flight' to cope with sudden stress |
Amines are also known for their unpleasant and often fishy smell.
Naming amines:
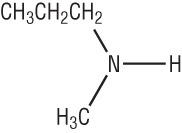
To the longest single alkyl chain add the suffix 'amine':
If there are 2 alkyl groups on the nitrogen:
 |
|
Basicity in amines:
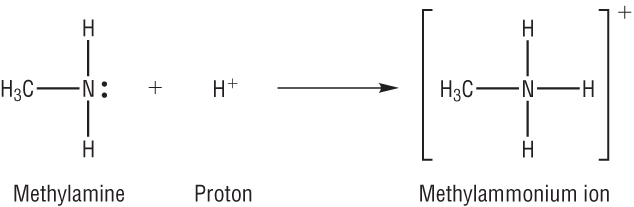
Amines are weak bases - proton acceptors.
This is because they have a lone pair of electrons on the nitrogen available to donate in accepting a hydrogen ion:
The inductive effect:
The basic properties of phenylamine are less than that of ammonia.
The basic properties of amines are greater than that of ammonia.
This can be explained by the inductive effect:
|
pH 8 |
Phenylamine |
|
|
pH 10 |
Ammonia |
NH3 |
|
pH 12 |
Butylamine |
CH3CH2CH2CH2NH2 |
Remember a base is a proton acceptor and the proton is accepted using the lone pair of electrons on the nitrogen.
Groups attached to the functional group can have an effect on how available the lone pair of electrons for accepting a proton:
|
|
Alkyl groups have a positive inductive effect. This means that they give a small push of electrons towards the neighboring atom (the nitrogen). This gives an increased electrons charge density meaning a better chance of the lone pair being used to accept a proton – Stronger base |
|
|
Ammonia has no inductive effect as there is nothing attached to the functional group. |
|
|
Benzene rings have a negative inductive effect. This means that the benzene ring has a small pull of electrons away from the neighboring atom (the nitrogen). This gives a lower electron charge density making it harder for the lone pair of electrons to be used to accept a proton – Weaker base
This would be further compounded by the lone pair of electrons on the nitrogen being able to delocalize into the benzene ring. |
|
|
Base reactions of amines:
Just as ammonia forms salts with acids so do amines:
|
|
Base | + | Acid | ŕ | Salt |
|
|
NH3(aq) | + | HCl(aq) | ŕ | NH4+Cl-(aq) |
|
|
C4H9NH2(aq) | + | HCl(aq) | ŕ | C4H9NH3+Cl-(aq) |
|
|
C6H5NH2(aq) | + | HCl(aq) | ŕ | C6H5NH3+Cl-(aq) |
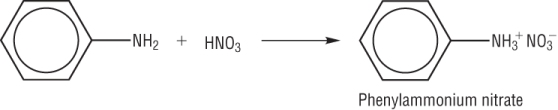
Questions 1 - 2 P37
Preparation of primary aliphatic amines:
These are made by warming halogenoalkanes with excess ammonia:
CH3CH2CH2Cl + NH3 ŕ CH3CH2CH2NH2 + HCl
The excess ammonia reacts with the HCl formed:
NH3 + HCl ŕ NH4Cl
Preparation of secondary / tertiary aliphatic amines:
Propylamine can react further (like the ammonia) with more chloropropane:
CH3CH2CH2Cl + CH3CH2CH2NH2 ŕ (CH3CH2CH2)2NH + HCl
And dipropylamine can react even further again:
CH3CH2CH2Cl + (CH3CH2CH2)2NH ŕ (CH3CH2CH2)3N + HCl
Multiple substitution is avoided by having ammonia in excess. This minimises the 'chance' of further substitution.
Preparation of aromatic amines:
Nitrobenzene (and other nitroarenes) can be reduced using a mixture of tin and concentrated hydrochloric acid:
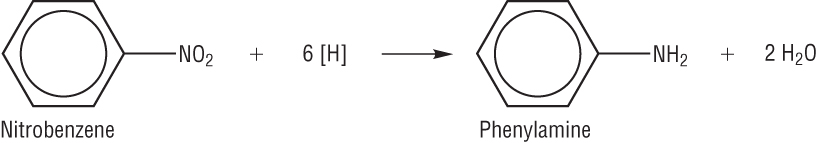
The excess HCl is neutralised at the end of the reaction.
This is an important reaction as it is used in the manufacture of dyes.
Synthesis of dyes from phenylamine:
There are 2 stages in this process:
1) Diazotisation
2) Coupling reactions
1) Diazotisation:
NaNO2 + HCl ŕ HNO2
Sodium nitrite Hydrochloric acid Nitrous acid
b) Diazotisation reaction:
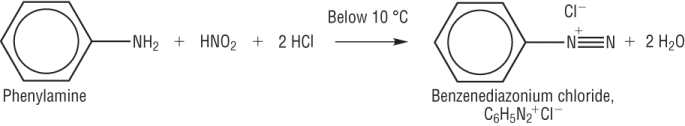
The diazonium ion, N2+ is unstable and decomposes releasing nitrogen, this is why it needs to be kept below 10oC.
The benzene ring in benzenediazonium salts allow the p electrons from the diazonium functional group to be delocalised over the benzene ring.
This stabilises the benzenediazonium salts enough to be used at low temperatures.
Questions 1 - 3 P39 Qu 9 P41 Qu 2a (ii) (iii) P42 Qu 11-13 P44,45
Have an NH2, amine and a COOH, carboxylic acid group:
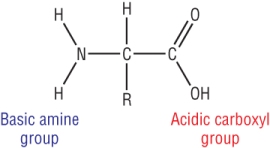
They are the building blocks for proteins which are held together by peptide links.
The body has 20 different amino acids which, as proteins can be enzymes, hormones, antibodies.
These proteins are responsible for body functions such as carrying oxygen in the blood, formation of bones etc.
The 20 Naturally occurring amino acids have the general formula of:
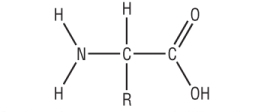
This arrangement is called a amino acids due to its optical isomerism (covered later)
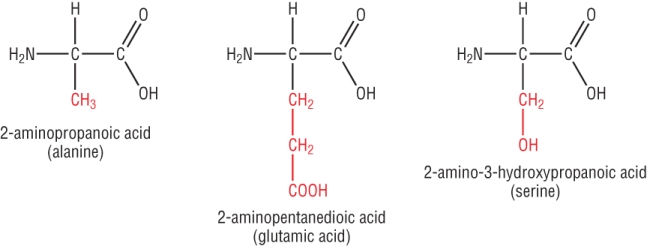
The R group can contain OH, SH, COOH or NH2 group.
Glycine is the simplest amino acid which means it will not contain an R group:
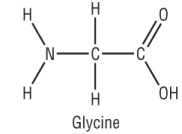
Other examples of a amino acids are shown below with different R groups attached:
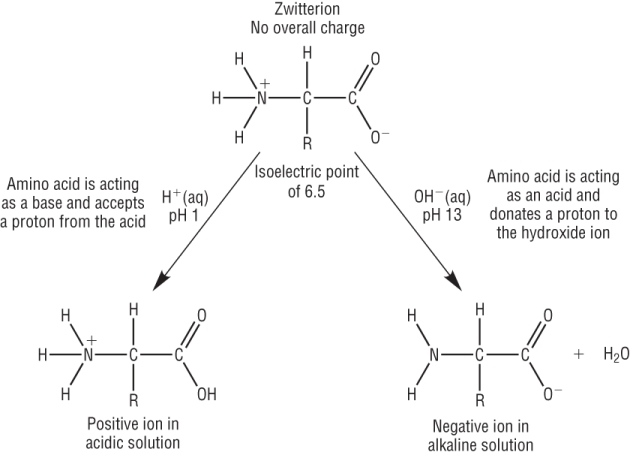
Zwitterion and the isoelectric point:
Amino acids contain an acidic carboxylic acid group and a basic amine group.
These can interact with each other to form a zwitterion:
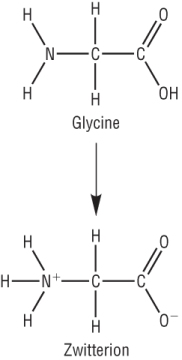 |
|
Comparison of melting points:
|
Molecule |
Melting point |
|
|
Glycine, |
NH2CH2COOH |
262oC |
|
Propanoic acid |
CH3CH2COOH |
-21oC |
Acid and base properties of amino acids:
Amino acids are amphoteric.
This means that they will react with both:
Acids: Due to the basic NH2 present.
Alkalis: Due to the acidic COOH present.
| pH < Isoelectric point: | pH > Isoelectric point: |
 |
|
|
|
Qu 1 - 3 P51
Amino acids and condensation reactions:
When 2 amino acids join together we call it a dipeptide.
When 3 amino acids join together we call it a tripeptide.
When many join together we call it a polypeptide.
Polypeptides are synthetic.
Proteins are natural and are usually larger than polypeptides.
The term ‘Peptide linkage’ (or bond) is the name for the amide link which in poly peptides or proteins.
The reaction is called a condensation reaction as water is given off:
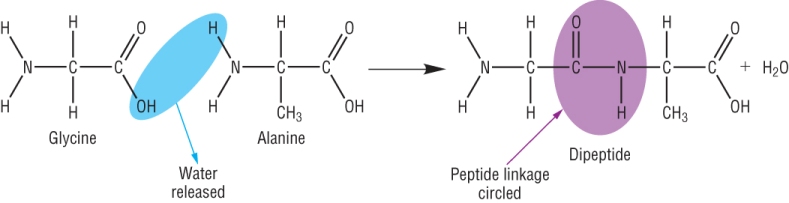
The functional group (in purple) is an amide group (CONH)
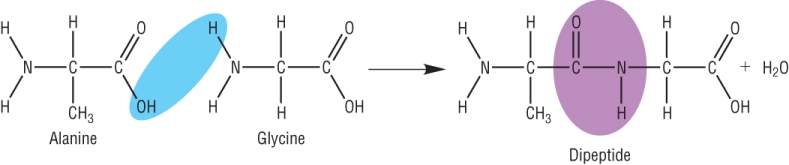
A different dipeptide can be made by joining them the other way round:
Forming polypeptides and proteins:
This is a long chain of amino acids joined by peptide linkages:
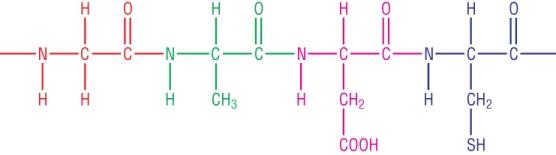
Each linkage forms a molecules of water.
A polypeptide generally has >50 amino acids.
Hydrolysis of polypeptides and proteins:
Polypeptides and proteins can be hydrolysed back into their constituent amino acids.
Acid hydrolysis:
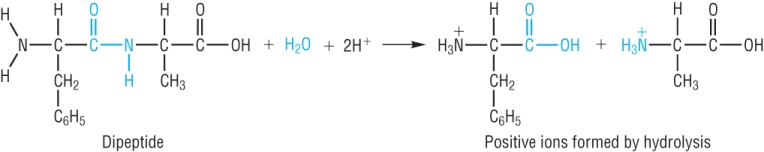
Alkali hydrolysis:
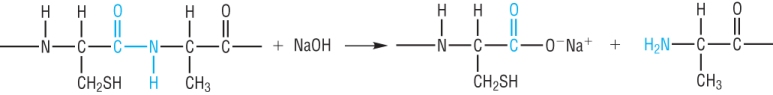
Qu 1 - 3 P51
Condensation polymerisation: 1) Polyesters
This is the joining of s monomers while eliminating a small molecule - H2O or HCl
The functional group on one monomer joins with a different functional group in another molecule.
There are 2 types of condensation polymerisations covered
Polyesters - from alcohols and carboxylic acids
Polyamides - from amines and carboxylic acids
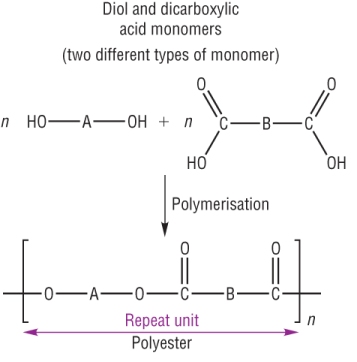
1) polyesters:
A polyester is made by condensing an alcohol and carboxylic acid.
The joining link is an ester functional group, hence polyester.
A polyester is can be made in one of 2 ways:
a) 2 monomers: Diol and Dicarboxylic acids-
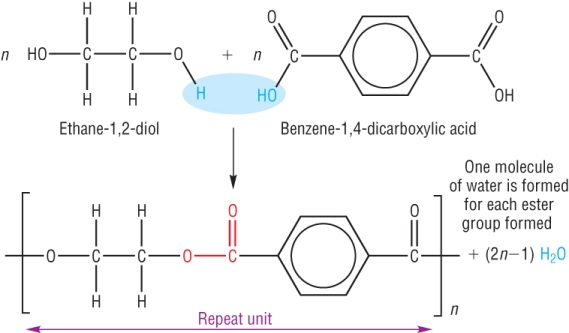
Terylene is made from the reaction between the monomers ethane-1,2-diol and benzene-1,4-dicarboxylic acid.
It is described as a condensation reaction as water is eliminated as the ester link is formed.
Used in the manufacture of carpets.
b) 1 monomer: Hydroxycarboxylic acid
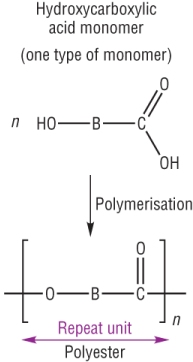
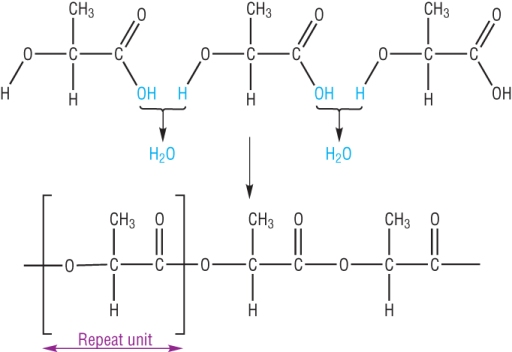
Poly(lactic acid), PLA is made from the reaction between the same monomer containing an OH and a COOH group.
It is described as a condensation reaction as water is eliminated as the ester link is formed.
Qu 1-3 P55
Condensation polymerisation: 2) Polyamides:
Polyamides are made by an amine / carboxylic acid condensation reaction.
The resulting link is an amide:
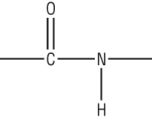
A polyester is can be made in one of 2 ways:
a) 2 monomers: Diamine and Dicarboxylic acids-
Formed from the reaction between the 2 monomers containing 2 NH2 and 2 COOH groups.
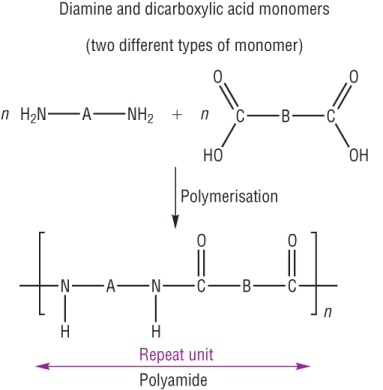
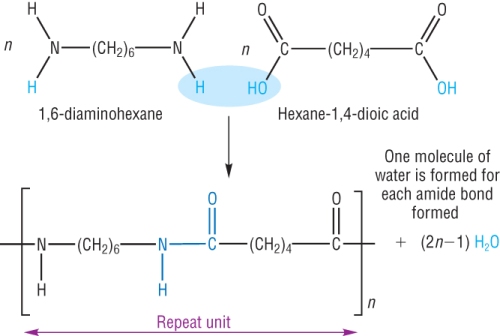
Nylon is made from the reaction between the monomers 1,6-diaminohexanel and hexane-1,4-dioic acid.
It is described as a condensation reaction as water is eliminated as the amide link is formed.
Used in the manufacture of clothing.
Another is kevlar - very strong polymer used in fire and bullet proof vest and crash helmets:
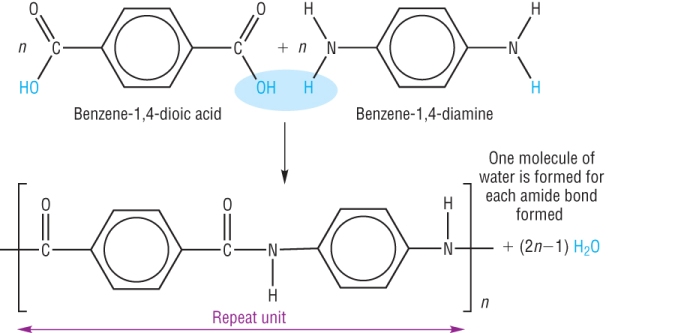
b) 1 monomer: Hydroxycarboxylic acid
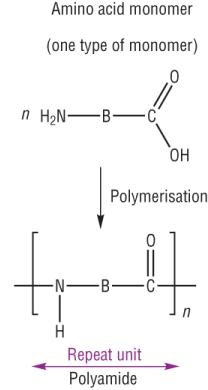
A monomer with an amine group at one end and a carboxylic acid group at the other.
 |
.jpg) |
Polypeptides and proteins - from the reaction between the same monomer containing an NH2 and a COOH group.
It is described as a condensation reaction as water is eliminated as the amide link is formed.
Qu 1-3 P57
Addition and condensation polymerisation
Addition polymers:
This was covered in AS under the alkenes chapter:
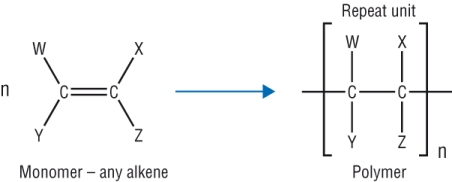
These are made from one monomer only - containing a C=C
Only one product is formed.
Using different alkene molecules, different addition polymers can be made:
.jpg)
Condensation polymerisation:
These polymers are when monomers are joined with the elimination of a small molecule, H2O or HCl.
The monomers must have 2 functional groups.
Comparison of addition and condensation polymers:
| Addition polymerisation | Condensation polymerisation | ||
| Polyester | Polyamide | ||
| Functional groups | C=C | COOH / OH | COOH / NH2 |
| No monomers | 1 | 1 or 2 | 1 or 2 |
| Products | poly(alkene) | polyester + water | polyamide + water |
| linkage | C-C |
 |
 |
Workes example P59
Qu 1,2 P59
Breaking down condensation polymers
If condensation polymerisation eliminates water then they can be hydrolysed with the addition of water (acid / base)
2 condensation polymers have been covered and both can be hydrolysed:
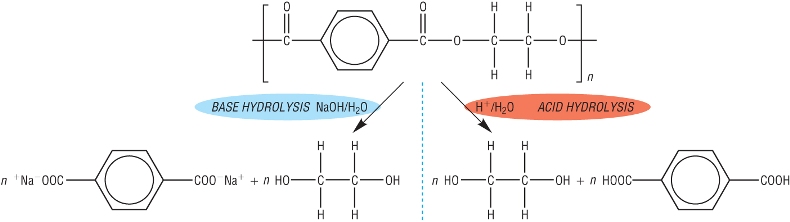
1) Hydrolysis of polyesters:
In carbonyl compounds we saw that esters can be hydrolysed in acidic or basic conditions.
This gave the corresponding alcohol and carboxylic acid (or salt of acid - base)
Polyesters can be hydrolysed in exactly the same way with hot aqueous acid / aqueous alkali.
The monomers making up the polymer are produced (or salt if base hydrolysis used):
2) Hydrolysis of polyamides:
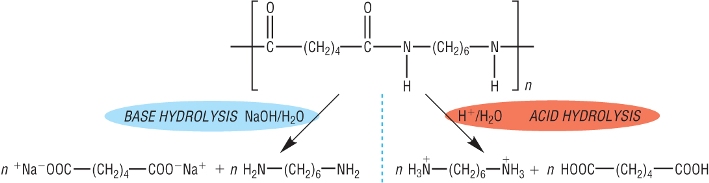
Polyesters can also be hydrolysed with hot aqueous acid / aqueous alkali.
The monomers making up the polymer are produced - ammonium in acidic conditions - carboxylate salts in basic:
Degradable polymers:
Most plastic packaging is addition polymers which will not degrade in landfill sites.
Environmental demand has produced biodegradable plastics.
These have chemical bonds that will hydrolyse similar to polyesters and polyamides.
A polymer based on tapiocha starch will decompose in 28 days when buried.
Photodegradable polymers:
These become weak and brittle when exposed to light.
The polymers are blended with light sensitive catalysts which will catalyse the decomposition of the polymer.
Another way is to encorperate C=O bonds within the polymer structure.
These absorb UV light and break.
Photodegradable plastics break to form waxy shorter hydrocarbon molecules before bacteria breaks them further into CO2 and H2O.
Qu 1-3 P61
Qu 9 P41 Qu 2a (ii) (iii) P42 Qu 11-13 P44,45
Qu 1,2,4,5,6 P 69 / Qu 1a,2,3,4,5,6,9,10,11,12 P70-73