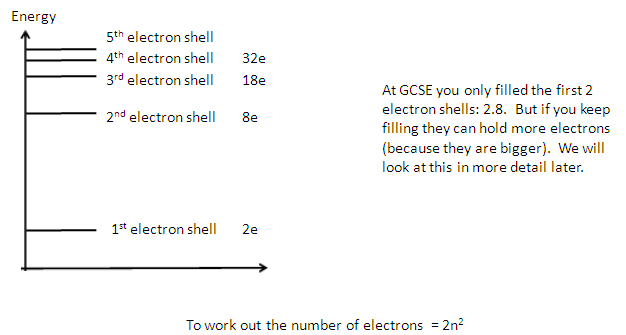
Evidence for shells
History of the atom
·
The model of the atom has changed as
our observations of its behaviour and properties have increased.
·
A model is used to explain
observations. The model changes to explain any new observations.
·
The stages in the
development of the atom:-
George Johnstone Stoney (1891)
Electrolysis. The charge of an electron.
Joseph J Thompson (1897)
The cathode ray tube and e/m deflection. The mass / charge of an electron.
Robert Milikan (1909)
Oil drop experiment. The mass / charge of an electron.
Joseph J Thompson
‘Plum – Pudding’ model of an atom.
Geiger, Rutherford and Marsden (1909)
Alpha particle deflection. The nuclear model.
Henry Moseley (1913)
Atomic number
Plasma displays
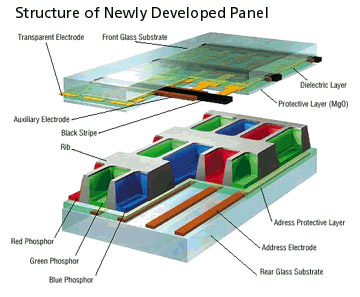 |
- A plasma unit has 100,000's of tiny cells
(pixels) filled with a mixture of neon and xenon gasses.
- A single pixel is made up of three coloured
sub-pixels, red, green and blue.
-
Each sub pixel is driven
by its own electrode, stimulates the gas to
release ultraviolet light photons.
-
The photons interact with
a phosphor material coated on the inside
wall of the cell.
-
Phosphors are substances
that give off light when they are exposed to
other light eg. Ultraviolet light. The
phosphors in a pixel give off coloured light
when they are charged.
- The cells are situated in a grid like
structure.
-
The varying intensity of
the current can create millions of different
combinations of red, green and blue across
the entire spectrum of colour.
|
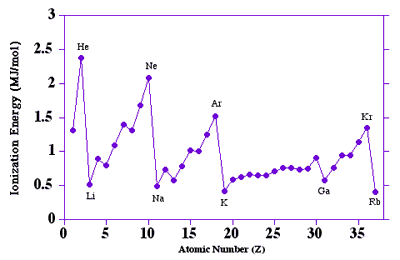
Ionisation energies: Click for DEMO
(click demo once)
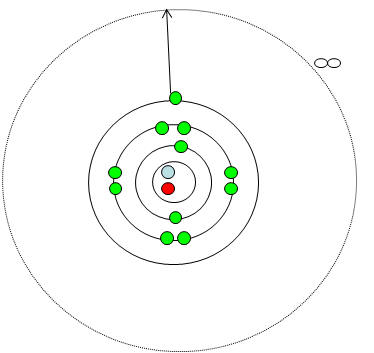 |
·
To form positive ions, electrons must
be completely removed i.e. ionisation.
·
To do this the electron must
completely escape the attraction of the atom. i.e. reach n=¥.
·
At n=¥
the electron has sufficient energy to escape the attraction from the nucleus.
|
Definition:
Factors affecting ionisation energy -
always refer to all 3 in any explainations:
1)
The distance of the electron
from the nucleus
F
a 1/d2
2)
Size of the positive nuclear
charge
-
The more protons in the nucleus,
the higher the nuclear charge, the harder it is to remove an electron, the
higher the ionisation energy.
3)
The ‘shielding’ effect by
full inner shells
-
A full inner shell of electrons
will repel electrons in outer shells.
-
These ‘shields’ affect of the
attraction from the nucleus
on outer electrons.
-
The more inner shells the
greater the shielding.
-
As
you go down a Group, the outer electron shell is further from the nucleus -
attraction decreases.
-
The more inner shells the
greater the shielding.
-
As you go down Group 2,
ionisation energy decreases.
-
As
you go across a Period, electrons are removed from the same electron shell,
shielding is the same.
-
This means that the No of
electrons : protons decreases.
-
This increases the attraction
pulling the electron shell in slightly.
-
This increases attraction.
-
This increases the successive
ionisation energies
Click for
SUMMARY DEMO
Questions: 1 - 3 p41/ 13 p73
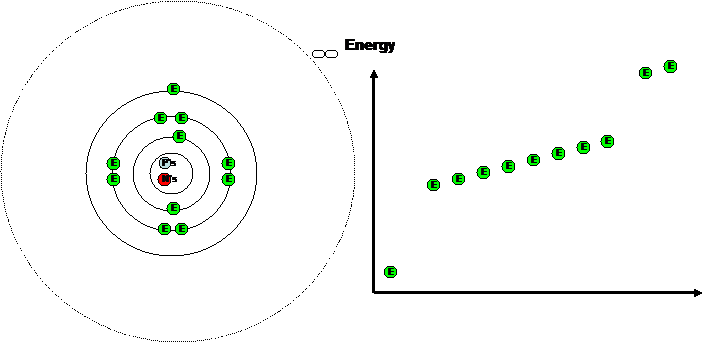
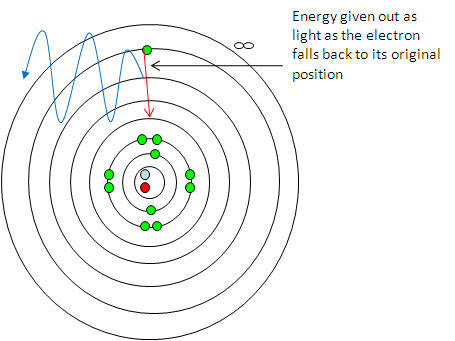
Shells and orbitals
Flame colours and emission spectra

See some line emission spectra
Energy levels or shells -
-
We have already seen what happens when an electron is
removed from an atom - the atom becomes and ion.
-
What if we supply enough energy to promote electrons to
higher empty electron shells
but not remove it?
-
One way to look at the arrangement of
electrons in an atom is to disturb them and see what happens when they go back
to their original arrangement.
-
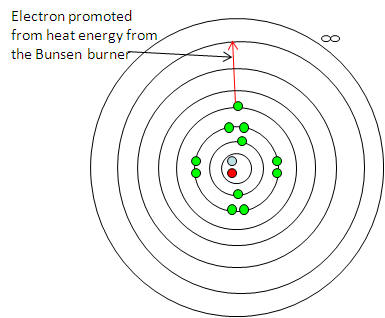
This is done by heating compounds in
a non-luminous Bunsen flame and studying the characteristic colours emitted when
the electrons fall back to their original arrangement.
-
The electrons gain energy from the
Bunsen and lose energy (as light ) as they go back to their original
arrangement:
-
This happens for any electron being promoted to any
higher electron shell and falling back to any lower electron shell.
-
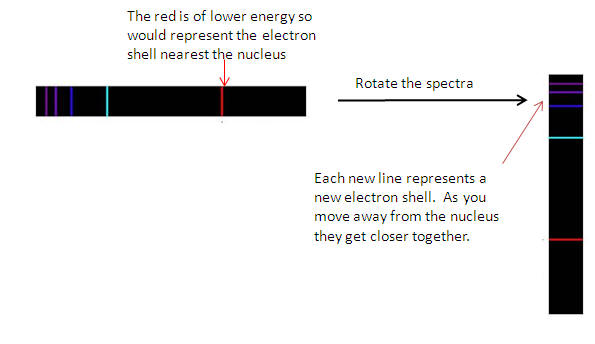
This gives us the series of lines as electrons give out
only specific energy when they move to a lower electron shell.
-
A spectroscope
shows us the specific energies characteristic to that element.
-
Looking at the line emission spectra 2 thing become
apparent:
1) That the lines are specific colours representing specific energies /
electron shells.
2) That as the move up in energy (towards the violet end) they get closer
together (converge)

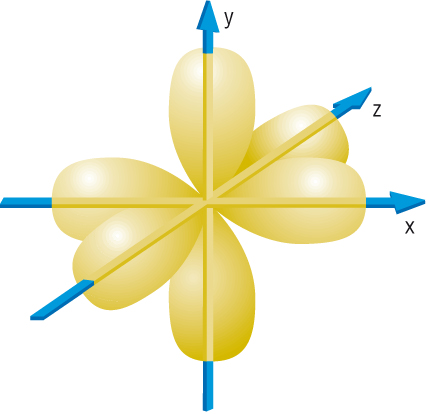
Atomic orbitals

-
At GCSE you will have been given a planetary model of
the atom. This model assumes electrons are solid particles.
-
Electrons are not solid particles, (more like a wave
packet of energy or electron cloud):
.jpg)
-
The very nature of the electron means that electrons
cannot orbit around around the nucleus.
-
Heisenberg's uncertainty principle states that
we cannot determine both position of the electron is and its
momentum.
-
This makes it impossible to determine the orbit
-
And so the planetary model has been replaced with the
orbital model.
-
These are regions around the nucleus in which the
electron is likely to be found.
-
Each electron shell is made up of orbitals.
-
Each orbital can hold a maximum of 2 electrons.
-
Imagine each electron as an electron cloud with the
shape of an orbital.
-
Each electron in an orbital would be an electron cloud
of the same shape.
-
2 electrons in an orbital would be the same shape
but twice as dense.
s - Orbitals:
.jpg)
p - Orbitals:
| Electron shell |
Principle Quantum Number |
Types of orbital |
No electrons in s shell |
No electrons in p shell |
No electrons in d shell |
No electrons in f shell |
Total |
| 1 |
|
|
|
|
|
|
|
| 2 |
|
|
|
|
|
|
|
| 3 |
|
|
|
|
|
|
|
| 4 |
|
|
|
|
|
|
|
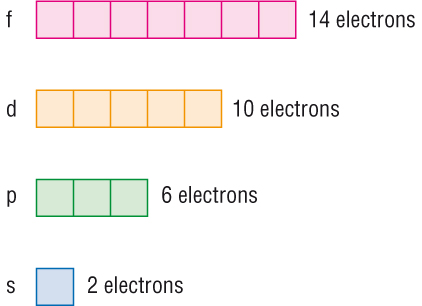
Representing electrons in orbitals
-
With different types of orbitals and having different
shapes, we represent an orbital with a box.
-
As a box is an orbital, each box can hold 2e.
-
When anything with a charge
spins a magnetic field is produced.
-
One of the electrons in an orbital will spin one way and the other spins in the opposite direction.
-
This gives opposing magnetic fields which we represent with an arrow.
-
This is the only way electrons can share an orbital as there is now some
attraction between them.


Questions 1 - 2 p43
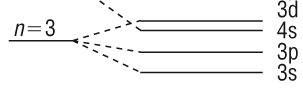
Sub - shells and energy levels
Sub - shells
-
An electron shell is
made up from orbitals.
-
Orbitals of the same
kind are called sub - shells.
-
This means that each
sub - shell contains the same type of orbitals, each of which holds a
maximum of 2e:
|
n = 1 shell: maximum 2
electrons |
| Sub - shell |
1s |
|
|
| Orbital |
 |
|
|
| Electrons |
2e |
|
|
| |
|
|
|
|
n = 2 shell: maximum 8
electrons |
| Sub - shell |
2s |
2p |
|
| Orbital |
 |
 |
|
| Electrons |
2e |
2e |
2e |
2e |
|
| |
|
|
|
|
n = 3 shell: maximum 18
electrons |
| Sub - shell |
3s |
3p |
3d |
| Orbital |
 |
 |
 |
| Electrons |
2e |
2e |
2e |
2e |
2e |
2e |
2e |
2e |
2e |
Electrons and the Periodic Table

-
After this the electrons have to pair up with those
spinning the opposite way.
-
In a sub-shell, electrons will remain unpaired in the
orbitals until they have to pair up.
-
This is the same in the d orbital.
-
-
-
-

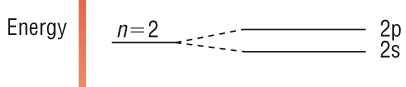
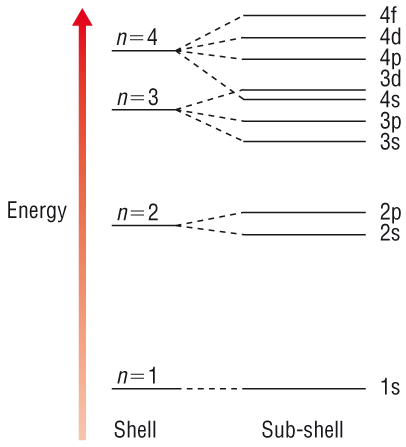
Electron energy
levels
-
Within an electron
shell, the sub - shells have slightly different energies as explained above.
-
The sub - shell
increases in energy in the order: s, p, d and f.
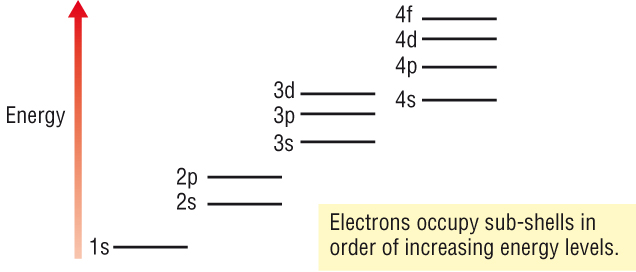
Filling shells and
sub - shells:
The Aufbau
Principle:
-
Electrons are added
one at a time to 'build up' the atom.
-
The lowest
available energy level fills first.
-
Each energy level
must be full before the next, higher energy level can be filled.
-
Each orbital in a
sub - shell is filled by single electrons before pairing up.
-
Each orbital can
hold 2e of opposite spin
Filling the
orbitals - Using the Aufbau Principle:
-
The first 3 electrons in
the p sub - shell spin in one direction and occupy px, py and
pz.
-
After this the electrons
have to pair up with those spinning the opposite way.
-
In a sub - shell, electrons
will remain unpaired in the orbitals until they have to pair up.
This is the same in the d orbital
Electron
configuration
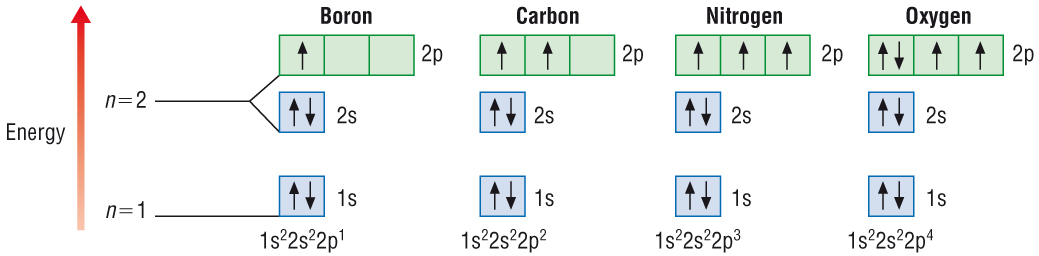
| Element |
Orbitals occupied |
Electron configuration |
| B |
1s22s22px1 |
1s22s22p1 |
| C |
1s22s22px12py1 |
1s22s22p2 |
| N |
1s22s22px12py12pz1 |
1s22s22p3 |
| O |
1s22s22px22py12pz1 |
1s22s22p4 |
Questions 1 - 2 p45
Sub shells and the Periodic Table:
| Element |
No electrons |
Electron configuration |
What Period is this element in? |
What is its highest Principle Quantum Number |
What is the highest sub - shell |
What Groups is the element in |
| H |
|
|
|
|
|
|
| He |
|
|
|
|
|
|
| Li |
|
|
|
|
|
|
| Be |
|
|
|
|
|
|
| B |
|
|
|
|
|
|
| C |
|
|
|
|
|
|
| N |
|
|
|
|
|
|
| O |
|
|
|
|
|
|
| F |
|
|
|
|
|
|
| Ne |
|
|
|
|
|
|
| Na |
|
|
|
|
|
|
| Mg |
|
|
|
|
|
|
| Al |
|
|
|
|
|
|
Things to notice:
-
The Period the element is in on
the Periodic Table is equivalent to its highest Principle Quantum Number, n
(and the electron shell of the outer electrons)
-
All s orbital elements are in
Groups 1 and 2 (except H and He)
-
All p orbital elements are in
groups 3,4,5,6,7 and 0
Extending further:
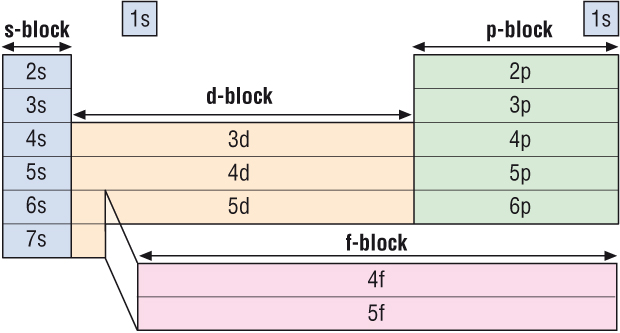
-
The Periodic Table is arranged in blocks of 2, 6, 10
and 14
-
s block elements are all in Gp1 and 2 = 2
-
p block elements are all in Gp3,4,5,6,7 and 0 = 6
-
d block elements are all in the Transition Metals =
10
-
f block elements are all in the Lanthanides and
Actinides = 14
Using the Periodic Table for electron configurations
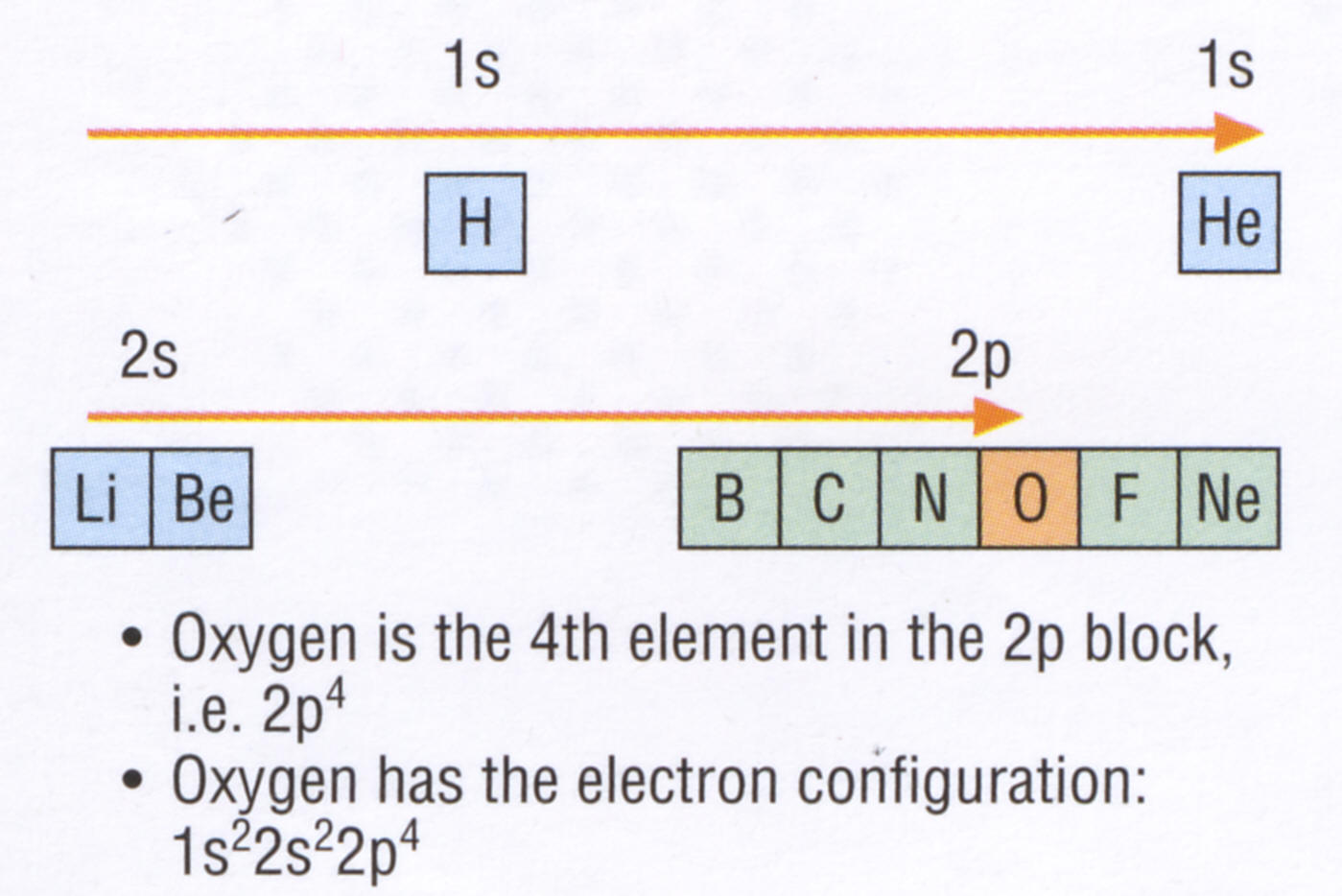
Other examples:-
1)
Na
1s2
2s2 2p6 3s1
2)
Sc
1s2
2s2 2p6 3s2 3p6 3d1 4s2
(remember 4s fills before 3d)
Shortening an electron configuration
Sc 1s2
2s2 2p6 3s2 3p6 3d1 4s2
Sc
[1s2
2s2 2p6 3s2 3p6]3d1 4s2
Inner shells
[Ar] has the same electron configuration as the inner shells
[Ar] 3d1 4s2
where [Ar] represents the electronic configuration of argon.
Electronic configurations in ions
·
Follow
the same principle as for atoms but add / remove electrons:-
Eg Sodium
ion Na+
Na atom = 1s2
2s2 2p6 3s1
To make a
sodium ion 1 electron is removed
Na ion = 1s2
2s2 2p6
Eg Chlorine ion Cl-
Cl atom = 1s2
2s2 2p6 3s22p5
To make a
chlorine ion 1 electron is added
Cl- ion = 1s2
2s2 2p6 3s22p6
Things to note:
Questions 1 - 4 p47 / 1,2 and 13
p73 / 1 p74








.jpg)